Praja2 controls P-body assembly and translation in glioblastoma by non-proteolytic ubiquitylation of DDX6
- PMID: 40148504
- PMCID: PMC12069581
- DOI: 10.1038/s44319-025-00425-5
Praja2 controls P-body assembly and translation in glioblastoma by non-proteolytic ubiquitylation of DDX6
Abstract
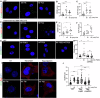
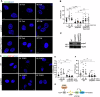
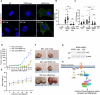
Glioblastoma multiforme (GBM) is the most lethal form of malignant brain tumor in adults. Dysregulation of protein synthesis contributes to cancer cell plasticity, driving GBM cell heterogeneity, metastatic behavior, and drug resistance. Understanding the complex network and signaling pathways governing protein translation, is therefore an important goal for GBM treatment. Here we identify a novel signaling network centered on the E3 ubiquitin ligase praja2 that controls protein translation in GBM. Praja2 forms a multimeric complex with the RNA helicase DDX6, which inhibits translation of target RNAs within processing bodies (P-bodies). Stimulation of cAMP signaling through activation of G-protein-coupled receptors induces P-body assembly through praja2-mediated non-proteolytic polyubiquitylation of DDX6. Genetic inactivation of praja2 reshapes DDX6/mRNA complexes and translating polysomes and promotes cellular senescence and GBM growth arrest. Expression of an ubiquitylation-defective DDX6 mutant suppresses the assembly of P-bodies and sustains GBM growth. Taken together, our findings identify a cAMP-driven network that controls translation in P-bodies and GBM growth.
Keywords: Glioblastoma; P-body; PKA; Praja2; cAMP.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures







References
-
- Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I (2015) Targeting the translation machinery in cancer. Nat Rev Drug Discov 14:261–278 - PubMed
-
- Bidwell P, Joh K, Leaver HA, Rizzo MT (2010) Prostaglandin E2 activates cAMP response element-binding protein in glioma cells via a signaling pathway involving PKA-dependent inhibition of ERK. Prostaglandins Other Lipid Mediators 91:18–29 - PubMed
MeSH terms
Substances
Grants and funding
- IG2023-29124/Fondazione AIRC per la ricerca sul cancro ETS (AIRC)
- E63C22000940007/Ministero dell'Università e della Ricerca (MUR)
- E53D23009690006/Ministero dell'Università e della Ricerca (MUR)
- E53D23021760001/Ministero dell'Università e della Ricerca (MUR)
- PE0000006/Ministero dell'Università e della Ricerca (MUR)
LinkOut - more resources
Full Text Sources
Medical

