Immune targeting and host-protective effects of the latent stage of Toxoplasma gondii
- PMID: 40148566
- PMCID: PMC11964939
- DOI: 10.1038/s41564-025-01967-z
Immune targeting and host-protective effects of the latent stage of Toxoplasma gondii
Abstract
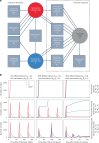
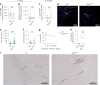
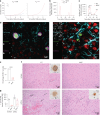
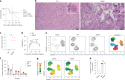
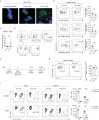
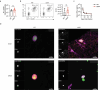
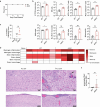
Latency is a microbial strategy for persistence. For Toxoplasma gondii the bradyzoite stage forms long-lived cysts critical for transmission, and its presence in neurons is considered important for immune evasion. However, the extent to which cyst formation escapes immune pressure and mediates persistence remained unclear. Here we developed a mathematical model highlighting that bradyzoite-directed immunity contributes to control of cyst numbers. In vivo studies demonstrated that transgenic CD8+ T cells recognized a cyst-derived antigen, and neuronal STAT1 signalling promoted cyst control in mice. Modelling and experiments with parasites unable to form bradyzoites (Δbfd1) revealed that the absence of cyst formation in the central nervous system did not prevent long-term persistence but resulted in increased tachyzoite replication with associated tissue damage and mortality. These findings suggest the latent form of T. gondii is under immune pressure, mitigates infection-induced damage and promotes survival of host and parasite.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Munz, C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat. Rev. Microbiol.17, 691–700 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

