A single centre experience of patients with rare cancers referred for early phase clinical trials
- PMID: 40148757
- PMCID: PMC11951660
- DOI: 10.1186/s12885-025-13934-2
A single centre experience of patients with rare cancers referred for early phase clinical trials
Abstract
Background: Cancers affecting < 6/100,000/year are classified as rare, but they account for up to 25% of all cancers and are associated with worse 5-year survival than common cancers. Early-phase clinical trials (EPCTs) may represent a viable treatment option for patients with rare cancers as they have evolved significantly with novel designs and the increasing use of precision medicine.
Methods: A retrospective study of patients with rare cancers referred to a large EPCT team at a UK specialist centre over 5 years (2016-2020) was conducted. Patient demographics, medical and oncological history, genomic variants, EPCT participation, responses and survival outcomes were analysed.
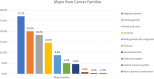
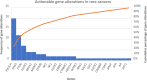
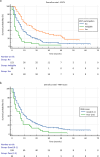
Results: In total, 240 patients with rare cancers were included. The mean age at diagnosis was 51.7 years (range 16-84), 54.2% of the patients were female. The most frequent rare cancers originated from the digestive system (27.1%), female genital tract (20%) and head and neck (H + N) (18.3%). Molecular profiling was offered to 45.5% of the population, median number of gene alterations was 3 per patient (range 1-20) while actionable gene alterations were reported in 60.2% (n = 50) of those with identified gene aberrations. Fifty-one patients participated in EPCTs, with 39.2% achieving SD and 11.8% PR. Median PFS for trial participants was three months (95% CI 1.12 - 4.88) while median OS in the trial patients was 16 months (95% CI 9.10 - 22.90) compared to 7 months for non-trial participants (95% CI 5.50 - 8.51). Finally, poor Royal Marsden Hospital (RMH) prognostic score (2-3) was correlated with worse survival when controlling for age and sex (HR 1.714, 95% CI 1.19 - 2.46, p = 0.004).
Conclusions: Participation of patients with rare cancers in EPCTs may be associated with a survival benefit and lead to the development of new treatments for these patients. Moreover, expanded use of precision medicine is paramount as it can inform targeted treatment selection in this heterogenous group.
Keywords: Early phase trials; Molecular profiling; Precision medicine; Prognosis; Rare cancers; Real world data; Targeted treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Overall approval as well as ethical approval for this study was received from the Quality Improvement and Clinical Audit Committee at the Christie NHS Foundation Trust for the study on 22nd April 2022 (reference 3299). As this was a retrospective study, consent to participate was not applicable and not required by the Quality Improvement and Clinical Audit Committee which provided approval for the project. Consent for publication: Not applicable. Competing interests: AA: None declared. NC: Financial Interests, Institutional, Invited Speaker: Roche, Taiho, AstraZeneca, RedX, Orion, Avacta, Bayer, Eisai, UCB, Starpharma, Boehringer Ingelheim, Stemline, Ergomed, LOXO Oncology, Nutide; Non-Financial Interests, Personal, Advisory Role: Roche. DMG: Financial Interests, Personal, Advisory Board, Consulting role on advisory board: Clinigen; Financial Interests, Personal, Invited Speaker: Cancer Drug Development Fund; Financial Interests, Personal, Advisory Board: McCann Health; Financial Interests, Institutional, Invited Speaker, Institutional funding from study: MSD, Codiak Biosciences, Starpharma, Faron Pharmaceuticals, Synthon, Janssen, Incyte; Financial Interests, Institutional, Research Grant: AstraZeneca. MK: Financial Interests, Personal, Advisory Board: Bayer, Roche, Janssen, Guardant Health; Financial Interests, Personal, Invited Speaker: Roche, Janssen; Financial Interests, Institutional, Expert Testimony: AstraZeneca; Financial Interests, Institutional, Advisory Board: Seattle Genetics; Financial Interests, Institutional, Invited Speaker: AstraZeneca, Blueprint, Astex, Bayer, BerGenBio, Carrick, Immutep, Janssen, Novartis, Nurix, Nuvalent, Pyramid Biosciences, Roche, Seattle Genetics, Turning Point Therapeutics; Financial Interests, Institutional, Research Grant: Roche, Novartis; Other, Personal, Other, Travel expenses for congress: Immutep, Janssen. FT: Financial interests, Personal, Advisory Board: BMS, CytomX, F-Star, Grey Wolf Therapeutics, GSK, Immatics, Leucid, Scenic Biotech, T-Knife Therapeutics; Financial interests, Personal, Invited Speaker: Kite Gilead; Financial interests, Personal, Other, Consultancy: Guidepoint; Financial interests, Institutional, Other: iMATCH director; Financial interests, Institutional, Local PI: Achilles ltd, Adaptimmune, Agalimmune Ltd, BMS, Crescendo, CytomX, GenMab, Immunocore, Incyte, Janssen, Novalgen, Novartis, Nucana, Oxford Vacmedix Ltd, Pfizer, RS Oncology LLC; Financial interests, Institutional, Coordinating PI: ADCT Therapeutics, Chugai, GSK, Instil Bio, Kymab Ltd/Sanofi, Roche; Financial interests, Institutional, Research Grant: GSK, Novartis, Autolus, Gilead; Non-financial interests, Institutional, Coordinating PI: T-Knife Therapeutics, AstraZeneca; Non-financial interests, Personal, Leadership role: Chair of the Independent Steering Committee for NIHR Blood & Transplant Research Unit, ESMO Congress 2024 Experimental Immunotherapy Track Chair, MRC Advanced Therapies Task Group, CRUK New Agents Committee Member, ESMO TAT conference scientific advisory board 2025, Sarcoma UK, Target Ovarian Cancer. LC: Financial Interests, Personal, Other, Consultancy: Bicycle Therapeutics, Boehringer Ingelheim, Athenex; Financial Interests, Personal, Full or part-time Employment, Medical Advisor: Cancer Research UK Centre for Drug Development; Financial Interests, Institutional, Invited Speaker: Boehringer Ingelheim, Bicycle Therapeutics, Cellcentric, Eli Lilly, Athenex, Lupin Limited, Repare Therapeutics, Cytomx therapeutics, EMD Serono/Merck KGaA, Sierra Oncology.
Figures
References
-
- Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet—a population-based study. Lancet Oncol. 2017;18(8):1022–39. - PubMed
-
- DeSantis CE, Kramer JL, Jemal A. The burden of rare cancers in the United States. CA Cancer J Clin. 2017;67(4):261–72. - PubMed
-
- Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–511. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

