Real-world use of difelikefalin in hemodialysis patients at a large dialysis organization in the United States: a retrospective database study
- PMID: 40148786
- PMCID: PMC11948630
- DOI: 10.1186/s12882-025-04074-7
Real-world use of difelikefalin in hemodialysis patients at a large dialysis organization in the United States: a retrospective database study
Erratum in
-
Correction: Real-world use of difelikefalin in hemodialysis patients at a large dialysis organization in the United States: a retrospective database study.BMC Nephrol. 2025 May 14;26(1):240. doi: 10.1186/s12882-025-04152-w. BMC Nephrol. 2025. PMID: 40369472 Free PMC article. No abstract available.
Abstract
Background: Chronic kidney disease-associated pruritus (CKD-aP) can negatively impact quality of life and survival among patients receiving maintenance hemodialysis. Difelikefalin, a selective κ-opioid receptor agonist, is the first medication approved for treatment of moderate-to-severe CKD-aP among patients on chronic hemodialysis. This retrospective database study assessed the real-world safety and effectiveness of difelikefalin across a large US dialysis organization.
Methods: We analyzed de-identified data from 715 adult hemodialysis patients treated with difelikefalin who had a Worst Itching Intensity Numerical Rating Scale (WI-NRS) score (0 = no itching to 10 = worst itch imaginable) assessed before therapy. Patients were classified as having received at least 30 difelikefalin doses over 12 weeks (complete regimen group; CRG) or fewer doses over that time period (incomplete regimen group; IRG). Mean baseline and follow-up WI-NRS scores were compared and potential adverse events evaluated.
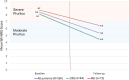
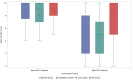
Results: Mean (SD) baseline WI-NRS scores were 8.5 (1.7), indicative of severe pruritic symptomatology. In the 22% of patients with follow-up data, mean WI-NRS scores improved by 2.9 points (8.4 [severe] to 5.4 [moderate]; P < 0.0001). This mean improvement was more pronounced in CRG patients (n = 84; 3.6) compared with IRG patients (n = 84; 2.2). Overall, 46% of patients experienced a 3-point reduction in itch severity. Difelikefalin initiation was not associated with changes in rates of nausea, diarrhea, vomiting, headache, or trouble walking. Dizziness and hyperkalemia were infrequent, but statistically significant with increases in dizziness (0.09% vs. 0.20%) and hyperkalemia (2.0% vs. 2.6%) were observed during treatment with difelikefalin.
Conclusions: In this analysis of real-world difelikefalin use in a US hemodialysis population, patients experienced significant reductions in CKD-aP, based on a validated measure of pruritus. Patients remaining on therapy for 12 weeks demonstrated greater symptom reductions than those patients receiving partial treatment. In combination with controlled trials, these data suggest that difelikefalin is an effective and well-tolerated treatment for the management of CKD-aP in adult patients receiving hemodialysis.
Keywords: Chronic kidney disease–associated pruritus; Difelikefalin; Hemodialysis; Symptoms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All data were extracted from the Fresenius Kidney Care clinical data warehouse and de-identified. The study was determined to be exempt under 45 CFR § 46.104(d)(4) by an independent institutional review board (New England Institutional Review Board [NEIRB], Needham, MA, USA; WCG IRB Work Order #1-1650918-1). Consent for publication: Not applicable. Competing interests: Linda H. Ficociello is employee of Renal Research Institute LLC, a wholly owned subsidiary of Fresenius Medical Care Holdings Inc. and has ownership interest in Fresenius Medical Care. Rachel Lasky is employee of Renal Research Institute LLC. Hans-Juergen Arens and Michael S. Anger are employees of and have stock/ownership interest in Fresenius Medical Care. Despina Ruessmann is an employee of and has ownership interest in CSL Vifor.
Figures
References
-
- Sukul N, Karaboyas A, Csomor PA, Schaufler T, Wen W, Menzaghi F, Rayner HC, Hasegawa T, Al Salmi I, Al-Ghamdi SMG, Guebre-Egziabher F, Ureña-Torres PA, Pisoni RL. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in Hemodialysis patients. Kidney Med. 2020;3(1):42–e531. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

