MAM kinases: physiological roles, related diseases, and therapeutic perspectives-a systematic review
- PMID: 40148800
- PMCID: PMC11951743
- DOI: 10.1186/s11658-025-00714-w
MAM kinases: physiological roles, related diseases, and therapeutic perspectives-a systematic review
Abstract
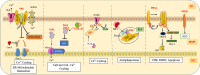
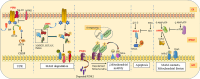
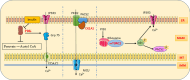
Mitochondria-associated membranes (MAMs) are tethering regions amid the membranes of the endoplasmic reticulum (ER) and mitochondria. They are a lipid raft-like structure occupied by various proteins that facilitates signal transduction between the two organelles. The MAM proteome participates in cellular functions such as calcium (Ca2+) homeostasis, lipid synthesis, ER stress, inflammation, autophagy, mitophagy, and apoptosis. The human kinome is a superfamily of homologous proteins consisting of 538 kinases. MAM-associated kinases participate in the aforementioned cellular functions and act as cell fate executors. Studies have proved the dysregulated kinase interactions in MAM as an etiology for various diseases including cancer, diabetes mellitus, neurodegenerative diseases, cardiovascular diseases (CVDs), and obesity. Several small kinase inhibitory molecules have been well explored as promising drug candidates in clinical trials with an accelerating impact in the field of precision medicine. This review narrates the physiological actions, pathophysiology, and therapeutic potential of MAM-associated kinases with recent updates in the field.
Keywords: Cancer; Diabetes; ER stress; Kinases; MAM; Mitophagy; Neurodegenerative disease; Therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable as this systematic review does not involve experimental procedures on human participants or animals. Consent of publication: Not applicable as this systematic review does not contain any person’s data in any form. Competing interests: The authors declare no competing interests.
Figures

References
-
- Barazzuol L, Giamogante F, Calì T. Mitochondria associated membranes (MAMs): architecture and physiopathological role. Cell Calcium. 2021. 10.1016/j.ceca.2020.102343. - PubMed
-
- Liu J, Yang J. Mitochondria-associated membranes: a hub for neurodegenerative diseases. Biomed Pharmacother. 2022. 10.1016/j.biopha.2022.112890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

