Decoding microglial immunometabolism: a new frontier in Alzheimer's disease research
- PMID: 40149001
- PMCID: PMC11948825
- DOI: 10.1186/s13024-025-00825-0
Decoding microglial immunometabolism: a new frontier in Alzheimer's disease research
Abstract
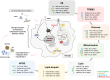
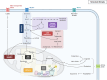
Alzheimer's disease (AD) involves a dynamic interaction between neuroinflammation and metabolic dysregulation, where microglia play a central role. These immune cells undergo metabolic reprogramming in response to AD-related pathology, with key genes such as TREM2, APOE, and HIF-1α orchestrating these processes. Microglial metabolism adapts to environmental stimuli, shifting between oxidative phosphorylation and glycolysis. Hexokinase-2 facilitates glycolytic flux, while AMPK acts as an energy sensor, coordinating lipid and glucose metabolism. TREM2 and APOE regulate microglial lipid homeostasis, influencing Aβ clearance and immune responses. LPL and ABCA7, both associated with AD risk, modulate lipid processing and cholesterol transport, linking lipid metabolism to neurodegeneration. PPARG further supports lipid metabolism by regulating microglial inflammatory responses. Amino acid metabolism also contributes to microglial function. Indoleamine 2,3-dioxygenase controls the kynurenine pathway, producing neurotoxic metabolites linked to AD pathology. Additionally, glucose-6-phosphate dehydrogenase regulates the pentose phosphate pathway, maintaining redox balance and immune activation. Dysregulated glucose and lipid metabolism, influenced by genetic variants such as APOE4, impair microglial responses and exacerbate AD progression. Recent findings highlight the interplay between metabolic regulators like REV-ERBα, which modulates lipid metabolism and inflammation, and Syk, which influences immune responses and Aβ clearance. These insights offer promising therapeutic targets, including strategies aimed at HIF-1α modulation, which could restore microglial function depending on disease stage. By integrating metabolic, immune, and genetic factors, this review underscores the importance of microglial immunometabolism in AD. Targeting key metabolic pathways could provide novel therapeutic strategies for mitigating neuroinflammation and restoring microglial function, ultimately paving the way for innovative treatments in neurodegenerative diseases.
Keywords: APOE; Aβ; HIF; Hexokinase; Immunometabolism; Metabolic reprogramming; Microglia; Neuroinflammation; TREM2; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Ferrari C, Sorbi S. The complexity of Alzheimer’s disease: an evolving puzzle. Physiol Rev. 2021;101:1047–81. - PubMed
-
- Jellinger KA. Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J Neural Transm (Vienna). 2022;129:1–24. - PubMed
-
- Al-Kuraishy HM, Jabir MS, Albuhadily AK, Al-Gareeb AI, Rafeeq MF. The link between metabolic syndrome and Alzheimer disease: a mutual relationship and long rigorous investigation. Ageing Res Rev. 2023;91:102084. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

