Carmofur Exhibits Antimicrobial Activity Against Streptococcus pneumoniae
- PMID: 40149043
- PMCID: PMC11939412
- DOI: 10.3390/antibiotics14030231
Carmofur Exhibits Antimicrobial Activity Against Streptococcus pneumoniae
Abstract
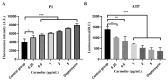
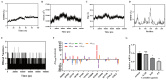
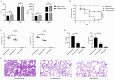
Background/Objectives:Streptococcus pneumoniae (S. pneumoniae) is a major pathogen causing severe infectious diseases, with an escalating issue of antimicrobial resistance that threatens the efficacy of existing antibiotics. Given the challenges in developing traditional antibiotics, drug repurposing strategies offer a novel approach to address the resistance crisis. This study aims to evaluate the antibacterial and anti-biofilm activities of the approved non-antibiotic anticancer drug carmofur against multidrug-resistant S. pneumoniae, and investigate the mechanism of action, and assess therapeutic potential in vivo. Methods/Results: Antimicrobial tests revealed that carmofur exhibited strong antibacterial activity against multidrug-resistant S. pneumoniae strains, with minimum inhibitory concentrations (MICs) ranging from 0.25 to 1 µg/mL. In the biofilm detection experiments, carmofur not only inhibited the formation of biofilms, but also effectively removed biofilms under high concentration conditions. Mechanistic studies showed that carmofur disrupted bacterial membrane permeability and decreased intracellular ATP levels. Molecular docking and dynamics simulation assays indicated that carmofur could stably bind to thymidylate synthase through hydrogen bonding and hydrophobic interactions, thereby exerting antibacterial effects. Meanwhile, carmofur was able to repress the expression of the thyA gene at the mRNA level. In a mouse infection model, the carmofur treatment group showed a reduction of approximately two log levels in bacterial load in lung tissue and blood, a significant decrease in the levels of inflammatory cytokines TNF-α and IL-6, and an improvement in survival rate to 60%. Conclusions: In summary, carmofur demonstrated significant antibacterial and anti-biofilm activities against multidrug-resistant S. pneumoniae and showed good anti-infective effects in vivo, suggesting its potential clinical application as a therapeutic agent against drug-resistant bacteria.
Keywords: S. pneumoniae; antibacterial; carmofur; mouse pneumonia; multidrug resistant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Repurposing High-Throughput Screening Identifies Unconventional Drugs with Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa under Experimental Conditions Relevant to Cystic Fibrosis.Microbiol Spectr. 2023 Aug 17;11(4):e0035223. doi: 10.1128/spectrum.00352-23. Epub 2023 Jun 12. Microbiol Spectr. 2023. PMID: 37306577 Free PMC article.
-
Antimicrobial polymers as therapeutics for treatment of multidrug-resistant Klebsiella pneumoniae lung infection.Acta Biomater. 2018 Sep 15;78:78-88. doi: 10.1016/j.actbio.2018.07.038. Epub 2018 Jul 20. Acta Biomater. 2018. PMID: 30031912
-
Antibacterial Properties and Efficacy of LL-37 Fragment GF-17D3 and Scolopendin A2 Peptides Against Resistant Clinical Strains of Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii In Vitro and In Vivo Model Studies.Probiotics Antimicrob Proteins. 2024 Jun;16(3):796-814. doi: 10.1007/s12602-023-10070-w. Epub 2023 May 6. Probiotics Antimicrob Proteins. 2024. PMID: 37148452
-
In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008-2010).Clin Infect Dis. 2012 Sep;55 Suppl 3:S206-14. doi: 10.1093/cid/cis563. Clin Infect Dis. 2012. PMID: 22903953 Review.
-
Treatment of Community-Acquired Pneumonia: A Focus on Lefamulin.Infect Dis Ther. 2021 Mar;10(1):149-163. doi: 10.1007/s40121-020-00378-3. Epub 2021 Feb 2. Infect Dis Ther. 2021. PMID: 33528794 Free PMC article. Review.
References
-
- Turner A.G., Ong C.Y., Walker M.J., Djoko K.Y., McEwan A.G. Transition Metal Homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae. Adv. Microb. Physiol. 2017;70:123–191. - PubMed
-
- Brueggemann A.B., Jansen van Rensburg M.J., Shaw D., McCarthy N.D., Jolley K.A., Maiden M.C.J., van der Linden M.P.G., Amin-Chowdhury Z., Bennett D.E., Borrow R., et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet. Digit. Health. 2021;3:e360–e370. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

