Sequential Treatment with Regorafenib and Trifluridine/Tipiracil ± Bevacizumab in Refractory Metastatic Colorectal Cancer in Community Clinical Practice in the USA
- PMID: 40149304
- PMCID: PMC11939964
- DOI: 10.3390/cancers17060969
Sequential Treatment with Regorafenib and Trifluridine/Tipiracil ± Bevacizumab in Refractory Metastatic Colorectal Cancer in Community Clinical Practice in the USA
Abstract
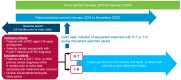
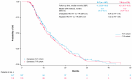
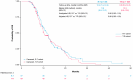
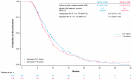
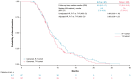
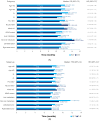
Background: Regorafenib (R) and Trifluridine/Tipiracil ± bevacizumab (T) are approved for treating refractory metastatic colorectal cancer (mCRC) but their optimal sequence is unclear. This study describes the characteristics/clinical outcomes of patients with mCRC in U.S. clinical practice treated sequentially with R-T or T-R. Methods: A retrospective cohort study of 818 patients treated with R-T or T-R between January 2015 and November 2022 was conducted using an electronic health record-derived database. The primary objective was to describe the demographic/clinical characteristics and biomarker status of patients treated with R-T or T-R, stratified by treatment line/age. Secondary objectives were to evaluate/estimate the frequency of neutropenia and myelosuppression-related treatments, the number/type of subsequent therapies, time to treatment discontinuation (TTD), and overall survival (OS). Results: Baseline characteristics were similar among patients who received R-T (n = 393) or T-R (n = 425). Lower rates of moderate/severe neutropenia (26%/12% vs. 32%/16%) and granulocyte colony-stimulating factor/erythropoietin use (22% vs. 24%) were observed with R-T versus T-R. The median TTD was 8.7 months and 8.5 months with R-T versus 8.1 months and 7.9 months with T-R as third- and fourth-line treatment, respectively. The median OS was 13.1 months and 11.6 months with R-T versus 11.5 months and 10.3 months with T-R as third- and fourth-line treatment, respectively. Conclusions: This study did not show a statistically significant difference in OS with R-T versus T-R. Although limited by its retrospective nature, the study suggested R-T may be preferable to T-R given the observed reduction in neutropenia/myelosuppression-related treatments.
Keywords: Regorafenib; TAS-102; Trifluridine/Tipiracil; colorectal cancer; community practice; metastatic; real world; refractory; sequence.
Conflict of interest statement
Milena Kurtinecz, Xiaoyun Pan, Zdravko Vassilev, Helene Ostojic, and Federica Pisa are employees of Bayer. Chengbo Yuan was an employee of Bayer at the time of the study. Tanios S. Bekaii-Saab has consulting or advisory roles with AbbVie, Amgen (Inst), Arcus Biosciences (Inst), AstraZeneca, Bayer (Inst), BeiGene, Boehringer Ingelheim, Celularity, Daiichi Sankyo/UCB Japan, Deciphera, Eisai, Exact Sciences, Foundation Medicine, Illumina, Immuneering, Incyte (Inst), Ipsen (Inst), Janssen, Kanaph Therapeutics, Lilly (Inst), Natera, Pfizer (Inst), Roche/Genentech (Inst), Seagen (Inst), SOBI, Stemline Therapeutics, Treos Bio, and other relationships with 1Globe Health Institute, AstraZeneca, Exelixis, FibroGen, Imugene, Lilly, Merck (Inst), Pancreatic Cancer Action Network, Replimune, Sun Biopharma, Suzhou Kintor Pharmaceuticals, UpToDate, and Xilis. Daniel Ahn has consulting or advisory roles with Exelixis, Genentech, and Novartis, and is a stockholder with Natera.
Figures
References
-
- National Cancer Institute Cancer Stat Facts: Colorectal Cancer. [(accessed on 9 December 2024)]; Available online: https://seer.cancer.gov/statfacts/html/colorect.html.
-
- Grothey A. Insights into the mechanism of action of Regorafenib in colorectal cancer. Clin. Adv. Hematol. Oncol. 2019;17((Suppl. S12)):2–4. - PubMed
-
- Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., Humblet Y., Bouche O., Mineur L., Barone C., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

