Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study
- PMID: 40149306
- PMCID: PMC11940497
- DOI: 10.3390/cancers17060972
Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study
Abstract
Background/objectives: Systemic treatment for unresectable hepatocellular carcinoma (HCC) has rapidly advanced, with immune checkpoint inhibitors now the preferred first-line option. However, with multiple agents available and no established treatment sequence, selecting the most suitable second-line (2L) therapy remains challenging. While sorafenib is frequently chosen for 2L treatment, comprehensive data supporting its use is limited. This study evaluates the effectiveness of sorafenib as 2L therapy and factors influencing outcomes following first-line treatment failure in advanced HCC patients.
Methods: This is a retrospective, multicenter study, including 81 patients with unresectable HCC from 12 European centers who received sorafenib as 2L treatment. Median overall survival (mOS), median progression-free survival (mPFS), radiological response to treatment, and toxicity were evaluated. Univariable and multivariable analyses were performed to identify potential predictors of clinical benefit.
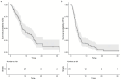
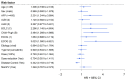
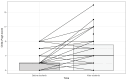
Results: In this cohort, some patients were treated with 2L sorafenib mOS for 7.4 months (95% CI: 6.6-13.6) and other patients were treated with mPFS for 3.7 months (95% CI: 3.0-4.8). Multivariable analysis revealed the best median OS for patients with CP A and AFP levels < 400 ng/mL (15.5 months). Adverse events (AE) of grade ≥ 3 were reported in 59.4% of patients.
Conclusions: In this real-world cohort of European patients with unresectable HCC, the outcome of sorafenib treatment in the 2L setting was comparable to that of the other established 2L treatment options in patients with preserved liver function and good performance status. This study contributes to the understanding of the role of sorafenib in the 2L setting and underscores the need for further research to identify predictive factors for response and survival in order to optimize treatment algorithms for advanced HCC.
Keywords: hepatocellular carcinoma; liver cancer; second-line therapy; sorafenib.
Conflict of interest statement
Author MP contributed to the advisory boards for AstraZeneca, Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; received speaker honoraria from Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; and received travel support from Bayer, BMS, Ipsen, and Roche. Author UE contributed to the advisory boards for AstraZeneca, Bayer, MSD, Roche, and Eisai; received speaker honoraria from AstraZeneca, Eisai, the FALK foundation, Ipsen, MSD, and Novartis; and received travel support from AstraZeneca and Biotest. Author AW contributed to the advisory boards for AstraZeneca, Bayer, BMS, MSD, Eisai, Servier, and Sanofi; received speaker honoraria from Leo Pharma, Eisai, Ipsen, Abbvie, AstraZeneca and Roche; and received travel support from Merck and Servier. Author AV contributed to the advisory boards for AstraZeneca, Amgen, BeiGene, Böhringer Mannheim, BMS, EISAI, Incyte, Ipsen, MSD, PierreFabre, Roche, Servier, and Tahio. MP received speaker honoraria from Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he is a consultant/advisory board member for AstraZeneca, Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he received grants from AstraZeneca, Bayer, BMS, Eisai, and Roche; and he received travel support from Bayer, BMS, Ipsen, and Roche. Author LR received consulting fees from AbbVie, AstraZeneca, Basilea, Bayer, BMS, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, and Zymeworks; lecture fees from AstraZeneca, Bayer, BMS, Guerbet, Incyte, Ipsen, Roche, and Servier; and travel expenses from AstraZeneca; Institutional research funding from Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, TransThera Sciences, and Zymeworks. Author MG contributed to the advisory boards for Roche, Eisai, MSD, BMS, AZ, and Servier. However, these activities have no potential conflicts of interest with the manuscript. None of the other authors have any potential conflicts (financial, professional, or personal) that are relevant to the manuscript. Autor EDT reports consultations for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, MSD, Mallinckrodt, Omega, Pfizer, IPSEN, Terumo, and Roche and employment at Boehringer-Ingelheim. He reports reimbursement of meeting attendance fees and travel expenses from Arqule, Astrazeneca, BMS, Bayer, Celsion, and Roche, and lecture honoraria from BMS and Falk. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, IPSEN, and Roche. Author AS received honoraria from BMS, Roche, Servier, Ipsen, Lilly, AstraZeneca, MSD, Eisai, and AMGEN. She received travel expenses from Servier, Pierre-Fabre, MSD, and Eisai and served on advisory boards for Eisai, MSD, Roche, Incyte, and BMS.
Figures



References
-
- Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- Faria S.C., Szklaruk J., Kaseb A.O., Hassabo H.M., Elsayes K.M. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: Concepts, perspectives, and radiologic implications. Abdom. Imaging. 2014;39:1070–1087. doi: 10.1007/s00261-014-0130-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

