Inherited Dyslipidemic Splenomegaly: A Genetic Macrophage Storage Disorder Caused by Disruptive Apolipoprotein E (APOE) Variants
- PMID: 40149441
- PMCID: PMC11942003
- DOI: 10.3390/genes16030289
Inherited Dyslipidemic Splenomegaly: A Genetic Macrophage Storage Disorder Caused by Disruptive Apolipoprotein E (APOE) Variants
Abstract
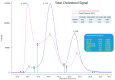
Background: Persistent splenomegaly, often an incidental finding, can originate from a number of inherited metabolic disorders (IMDs). Variants of APOE are primarily known as risk factors in terms of cardiovascular disease; however, severe dysfunction of APOE can result in a disease phenotype with considerable overlap with lysosomal storage disorders (LSDs), including splenomegaly and gross elevation of N-palmitoyl-O-phosphocholine-serine (PPCS).
Methods: A case study (deep phenotyping, genetic and FACS analysis) and literature study was conducted.
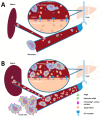
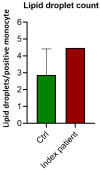
Results: The index patient, with a family history of early-onset cardiovascular disease, presented with splenic infarctions in a grossly enlarged spleen. The identified genetic cause was homozygosity for two APOE variants (c.604C>T, p.(Arg202Cys) and c.512G>A, p.(Gly171Asp); ε1/ε1), resulting in a macrophage storage phenotype resembling an LSD that was also present in the brother of the index patient. A FACS analysis of the circulating monocytes showed increased lipid content and the expression of activation markers (CD11b, CCR2, CD36). This activated state enhances lipoprotein intake, which eventually converts these monocytes/macrophages into foam cells, accumulating in tissues (e.g., spleen and vascular wall). A literature search identified seven individuals with splenomegaly caused by APOE variants (deletion of leucine at position 167). The combined data from all patients identified male gender, splenectomy and obesity as potential modifiers determining the severity of the phenotype (i.e., degree of triglyceride increase in plasma and/or spleen size). Symptoms are (partially) reversible by lipid-lowering medication and energy restricted diets and splenectomy is contra-indicated.
Conclusions: Inherited dyslipidemic splenomegaly caused by disruptive APOE variants should be included in the differential diagnoses of unexplained splenomegaly with abnormal lipid profiles. A plasma lipid profile consistent with dysbetalipoproteinemia is a diagnostic biomarker for this IMD.
Keywords: N-palmitoyl-O-phosphocholine-serine (PPCS); apolipoprotein E; dysbetalipoproteinemia; inherited lipemic splenomegaly; splenomegaly.
Conflict of interest statement
The authors report no financial or non-financial conflicts of interest related to this work.
Figures

References
-
- Rajkumar V., Dumpa V. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Lysosomal Storage Disease. - PubMed
-
- Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. - DOI - PMC - PubMed
-
- Voorink-Moret M., Goorden S.M.I., van Kuilenburg A.B.P., Wijburg F.A., Ghauharali-van der Vlugt J.M.M., Beers-Stet F.S., Zoetekouw A., Kulik W., Hollak C.E.M., Vaz F.M. Rapid screening for lipid storage disorders using biochemical markers. Expert center data and review of the literature. Mol. Genet. Metab. 2018;123:76–84. doi: 10.1016/j.ymgme.2017.12.431. - DOI - PubMed
-
- Dang Do A.N., Chang I.J., Jiang X., Wolfe L.A., Ng B.G., Lam C., Schnur R.E., Allis K., Hansikova H., Ondruskova N., et al. Elevated oxysterol and N-palmitoyl-O-phosphocholineserine levels in congenital disorders of glycosylation. J. Inherit. Metab. Dis. 2023;46:326–334. doi: 10.1002/jimd.12595. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

