Cerebral Amyloid Angiopathy: Clinical Presentation, Sequelae and Neuroimaging Features-An Update
- PMID: 40149580
- PMCID: PMC11939913
- DOI: 10.3390/biomedicines13030603
Cerebral Amyloid Angiopathy: Clinical Presentation, Sequelae and Neuroimaging Features-An Update
Abstract
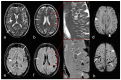
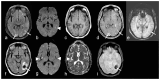
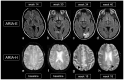
The prevalence of cerebral amyloid angiopathy (CAA) has been shown to increase with age, with rates reported to be around 50-60% in individuals over 80 years old who have cognitive impairment. The disease often presents as spontaneous lobar intracerebral hemorrhage (ICH), which carries a high risk of recurrence, along with transient focal neurologic episodes (TFNE) and progressive cognitive decline, potentially leading to Alzheimer's disease (AD). In addition to ICH, neuroradiologic findings of CAA include cortical and subcortical microbleeds (MB), cortical subarachnoid hemorrhage (cSAH) and cortical superficial siderosis (cSS). Non-hemorrhagic pathologies include dilated perivascular spaces in the centrum semiovale and multiple hyperintense lesions on T2-weighted magnetic resonance imaging (MRI). A definitive diagnosis of CAA still requires histological confirmation. The Boston criteria allow for the diagnosis of a probable or possible CAA by considering specific neurological and MRI findings. The recent version, 2.0, which includes additional non-hemorrhagic MRI findings, increases sensitivity while maintaining the same specificity. The characteristic MRI findings of autoantibody-related CAA-related inflammation (CAA-ri) are similar to the so-called "amyloid related imaging abnormalities" (ARIA) observed with amyloid antibody therapies, presenting in two variants: (a) vasogenic edema and leptomeningeal effusions (ARIA-E) and (b) hemorrhagic lesions (ARIA-H). Clinical and MRI findings enable the diagnosis of a probable or possible CAA-ri, with biopsy remaining the gold standard for confirmation. In contrast to spontaneous CAA-ri, only about 20% of patients treated with monoclonal antibodies who show proven ARIA on MRI also experience clinical symptoms, including headache, confusion, other psychopathological abnormalities, visual disturbances, nausea and vomiting. Recent findings indicate that treatment should be continued in cases of mild ARIA, with ongoing MRI and clinical monitoring. This review offers a concise update on CAA and its associated consequences.
Keywords: Boston criteria; amyloid-related imaging abnormalities; antibody treatment; cerebral amyloid angiopathy; inflammation; intracerebral hemorrhage; magnetic resonance imaging; sequelae.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

