Inhibition of DPP-4 Attenuates Endotoxemia-Induced NLRC4 Inflammasome and Inflammation in Visceral Adipose Tissue of Mice Fed a High-Fat Diet
- PMID: 40149869
- PMCID: PMC11940500
- DOI: 10.3390/biom15030333
Inhibition of DPP-4 Attenuates Endotoxemia-Induced NLRC4 Inflammasome and Inflammation in Visceral Adipose Tissue of Mice Fed a High-Fat Diet
Abstract
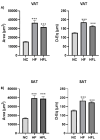
Inflammasomes are protein complexes that trigger pro-inflammatory responses and promote many diseases, including adipose tissue dysfunction. Linagliptin (L), a DPP-4 inhibitor used for type 2 diabetes therapy, has putative anti-inflammatory effects. This work explores L effects on inflammasome regulation, inflammation, and adipose tissue dysfunction in obese mice. Male C57BL/6N mice were fed a normal chow (NC) diet, high-fat (HF) diet, or HF diet with L (HFL) for 15 weeks. Gene expression and histological examinations were performed on visceral (VAT) and subcutaneous (SAT) adipose tissue samples. Biomarkers were quantified on sera. Murine macrophages were utilized for in vitro analyses. L decreased HF-induced endotoxemia and circulating inflammatory indicators. Despite having no effect on body weight, L reduced VAT inflammation by decreasing endotoxemia-induced NLRC4 inflammasome, inflammation severity, and fat cell hypertrophy. Although SAT response differed from VAT, inflammation was slightly reduced in this tissue too. In vitro, L modulated inflammation by directly reducing the pro-inflammatory macrophage phenotype. In obesity, increased NLRC4 inflammasome expression links endotoxemia and VAT inflammation. L protected against endotoxemia, maybe by affecting gut permeability and VAT responses. The decreased polarization of macrophages toward a pro-inflammatory phenotype and the reduction in adipocyte hypertrophy are involved in the response to L.
Keywords: DPP-4; NLRC-4 inflammasome; adipocytes; endotoxemia; fat; high-fat diet; inflammasomes; inflammation; linagliptin; macrophage phenotype.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Vianello E., Dozio E., Arnaboldi F., Marazzi M.G., Martinelli C., Lamont J., Tacchini L., Sigruner A., Schmitz G., Corsi Romanelli M.M. Epicardial adipocyte hypertrophy: Association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr. Metab. Cardiovasc. Dis. 2016;26:246–253. doi: 10.1016/j.numecd.2015.12.005. - DOI - PubMed
-
- Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. - DOI - PubMed
-
- Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S., Wang Z.V., Landskroner-Eiger S., Dineen S., Magalang U.J., et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

