Cetuximab and Paclitaxel Drug Response in Head and Neck Tumor Stem Cells
- PMID: 40149887
- PMCID: PMC11940455
- DOI: 10.3390/biom15030352
Cetuximab and Paclitaxel Drug Response in Head and Neck Tumor Stem Cells
Abstract
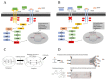
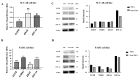
Head and neck cancer (HNC) is one of the most common types of cancer in the world, characterized by resistance to conventional therapies and an unfavorable prognosis due to the presence of tumor stem cells (TSCs). TSCs are cell subpopulations with high potential for invasion, migration, and metastasis, being responsible for the initiation and dissemination of cancer. This study aimed to evaluate the efficacy of treatments with cetuximab and paclitaxel, alone and in combination, in TSCs from oral cavity (SCC-28) and hypopharynx (FADU) cancer cell lines. In addition, the influence of the gene and protein expression of EGFR, NTRK2 (TRKB), KRAS, and HIF-1α on the response to treatments was investigated. TSCs were identified based on ALDH staining, and cell viability assays (MTS) indicated that both TSCs and non-TSCs showed resistance to cetuximab monotherapy, while paclitaxel, either alone or in combination with cetuximab, was more effective in reducing cell viability. Real-time PCR and Western blot analysis revealed increased expression of KRAS and HIF-1α in TSCs, suggesting their possible association with treatment resistance. The results of this study point to specific molecular factors that influence therapeutic responses in HNC, with an emphasis on the efficacy of drug combinations to overcome TSC resistance. The identification of these molecular mechanisms may provide guidelines for the development of more targeted and effective therapies against HNC, improving clinical management and patient prognoses.
Keywords: cancer treatment; cell signaling pathways; chemotherapy; gene expression; head and neck cancer; tumor stem cells.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Figures



References
-
- OMS Cancer Tomorrow. 2022. [(accessed on 6 January 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype.
-
- Head and Neck Cancer-National Cancer Institute. [(accessed on 12 January 2023)];2022 Available online: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet#:~:text=....
-
- Bian S., Wang Z., Chen Y., Li R. SPLUNC1 and MLL3 regulate cancer stem cells in nasopharyngeal carcinoma. J. BUON. 2019;24:1700–1705. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

