Synthesis, Antiproliferative Activity, and ADME Profiling of Novel Racemic and Optically Pure Aryl-Substituted Purines and Purine Bioisosteres
- PMID: 40149888
- PMCID: PMC11940194
- DOI: 10.3390/biom15030351
Synthesis, Antiproliferative Activity, and ADME Profiling of Novel Racemic and Optically Pure Aryl-Substituted Purines and Purine Bioisosteres
Abstract
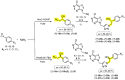
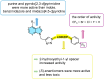
The aim of this study was to synthesize new racemic and optically pure aryl-substituted purine bioisosteres using ultrasound-assisted Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition. Regioselective synthesis of α-azido alcohols was applied to afford heterocycles with a 2-hydroxyeth-1-yl linker. Catalytic asymmetric synthesis using halohydrin dehalogenase in the ring-opening of epoxides gave enantioenriched azido alcohols, which subsequently afforded R- and S-enantiomers of purine and pyrrolo[2,3-d]pyrimidines with a 1-hydroxyeth-2-yl linker. The newly synthesized compounds were evaluated in vitro for their antiproliferative activity against four malignant tumor cell lines. The influence of regioisomerism and the stereochemistry of the hydroxyethyl group, as well as a N-heterocyclic scaffold linked to the aryl moiety on cytostatic activity was evaluated. Of all the compounds tested, purine 40a and pyrrolo[2,3-d]pyrimidine 45a derivatives with p-trifluoromethyl-substituted aryl connected to 1,2,3-triazole via a 2-hydroxyeth-1-yl spacer showed promising submicromolar antiproliferative activity. In addition, compound 45a exhibited selectivity towards the tumor cell line, with a selectivity index (SI) of 40, moderate clearance, and good membrane permeability.
Keywords: ADME profiling; antiproliferative activity; purine; purine bioisosteres.
Conflict of interest statement
Although the authors Sanja Koštrun and Astrid Milić are employees of Selvita d.o.o., they declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The remaining authors also declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

