Electrostatic Effects on Tau Nanocondensates
- PMID: 40149942
- PMCID: PMC11940141
- DOI: 10.3390/biom15030406
Electrostatic Effects on Tau Nanocondensates
Abstract
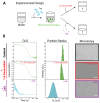
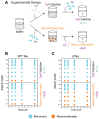
Biomolecular condensates (BMCs) are membrane-less protein compartments with physiological and pathological relevance. The formation of BMCs is driven by a process known as liquid-liquid phase separation (LLPS), a field that has largely focused on the study of micron-sized condensates. However, there have been recent studies showing that proteins that undergo LLPS also form nanometer-sized condensates. These nanometer-sized condensates, or nanocondensates, are distinct from microcondensates and potentially exhibit more relevance in cell biology. The field of nanocondensate research is in its infancy, with limited biophysical studies of these structures. Here, we studied condensate formation and dissolution of wild-type and disease-linked (hyperphosphorylated and missense mutated) Tau. We investigated the effects of solution condition modulation on nanocondensate formation and dissolution, and observed that Tau condensation is strongly regulated by electrostatic forces and less affected by hydrophobic disruption. We observed that all three Tau variants studied shared condensate formation properties when in solution conditions with the same ionic strength. However, hyperphosphorylated and missense-mutated Tau exhibited higher resistance to dissolution compared to wild-type Tau. This study uncovers additional distinctions between different types of condensates, which provides further insight into the distinctions between physiological and pathological condensates.
Keywords: LLPS; Tau; biomolecular condensates; nanocondensates; neurodegeneration; protein condensation; tauopathy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Longfield S.F., Mollazade M., Wallis T.P., Gormal R.S., Joensuu M., Wark J.R., van Waardenberg A.J., Small C., Graham M.E., Meunier F.A., et al. Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles. Nat. Commun. 2023;14:7277. doi: 10.1038/s41467-023-43130-4. - DOI - PMC - PubMed
-
- Siahaan V., Tan R., Humhalova T., Libusova L., Lacey S.E., Tan T., Dacy M., Ori-McKenney K.M., McKenney R.J., Braun M., et al. Microtubule lattice spacing governs cohesive envelope formation of tau family proteins. Nat. Chem. Biol. 2022;18:1224–1235. doi: 10.1038/s41589-022-01096-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

