Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection
- PMID: 40150063
- PMCID: PMC11940921
- DOI: 10.3390/diagnostics15060720
Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection
Abstract
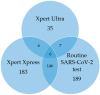
Background/Objectives: Tuberculosis (TB) and SARS-CoV-2 share similar symptoms and transmission routes. In early 2021, USAID and Stop TB Partnership recommended an integrated approach for simultaneous COVID-19 and TB testing in high TB burden countries for individuals with respiratory symptoms. In this evaluation, we tested a single sputum for both SARS-CoV-2 and Mycobacterium tuberculosis complex (MTBC) from participants at two healthcare facilities in South Africa. The diagnostic accuracy of the Xpert Xpress SARS-CoV-2 (Xpress) assay using a sputum swab capture method was assessed by comparing the results with routine SARS-CoV-2 testing, while also determining the prevalence of TB and TB-COVID-19 co-infection in the study population. Methods: A total of 2274 individuals were screened for enrolment. Eligibility included the presence of respiratory symptoms, close contact with a person with TB, TB diagnosis in the last two years or a person living with HIV. Sputum from 1032 participants was tested on the Xpress assay using a swab capture method while residual sputum was tested on the Xpert MTB/RIF Ultra assay for MTBC and rifampicin-resistance detection. Concordance between the Xpress assay and routine SARS-CoV-2 testing was assessed. Results: The Xpress assay detected SARS-CoV-2 in 183/1032 (18%) participants, TB was detected in 35/1032 (3%) participants and 10/1032 (1%) participants were co-infected with TB and COVID-19. The Xpress assay showed substantial agreement with routine testing (Kappa: 0.755). Conclusions: The study findings underscore a substantial identification of TB and rifampicin-resistant TB that would have been missed if bi-disease testing was not performed. In addition, the sputum swab capture method demonstrated reliable performance for SARS-CoV-2 detection.
Keywords: COVID-19; Mycobacterium tuberculosis; SARS-CoV-2; bi-disease testing; tuberculosis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Global Tuberculosis Report 2021. World Health Organization. [(accessed on 4 May 2022)]. Available online: https://www.who.int/publications/i/item/9789240037021.
-
- Global Tuberculosis Report 2024. World Health Organization. [(accessed on 4 November 2024)]. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa....
-
- Global Tuberculosis Report 2022. World Health Organization. [(accessed on 19 November 2022)]. Available online: https://www.who.int/publications/i/item/9789240061729.
-
- Global Tuberculosis Report 2023. World Health Organization. [(accessed on 19 December 2023)]. Available online: https://www.who.int/publications/i/item/9789240083851.
-
- National TB Recovery Plan. 2022. [(accessed on 5 November 2022)]; Available online: https://tbthinktank.org/wp-content/uploads/2022/09/TB-Recovery-Plan-for-....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

