The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis
- PMID: 40150124
- PMCID: PMC11941088
- DOI: 10.3390/diagnostics15060782
The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis
Abstract
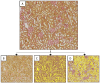
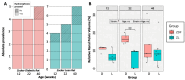
Background/Objectives: Diabetic kidney disease (DKD) is a significant concern for global healthcare, particularly in individuals with diabetes. The Zucker rat strain is a commonly used model of type 2 diabetes, despite awareness that this animal can develop hydronephrosis. In this study, we present novel imaging data evaluating the accuracy of this animal model in replicating the vascular aspects of human DKD while examining the impact of hydronephrosis on its validity as a disease model. Methods: This study reused data from a population of male Zucker Diabetic Fatty (ZDF; n = 22) rats and Zucker Lean (ZL) rats (n = 22) aged 12 to approximately 40 weeks. Vascular casting was performed to enable visualisation of the renal vasculature. Anatomical regional volumes and vascular density data were obtained from μCT scans using image thresholding and manual analysis. The effects of hydronephrosis were evaluated using renal functional parameters and histological examination. Results: A significantly lower cortical vascular density, as well as lower total renal vascular density, was seen in ZDF rats compared to ZL rats, independent of age. We identified that hydronephrosis affected 92% of ZDF rats and 69% of ZL rats. Hydronephrosis cavity size was significantly correlated with the degree of hyperglycaemia and rate of diuresis but had no other detected impact on renal function, vascularity, or tissue histological architecture. Conclusions: These findings support using the Zucker rat strain as a model for vascular changes in DKD. Despite identifying severe hydronephrosis in this population, it had minimal quantifiable impact on renal function or diabetes modelling.
Keywords: Zucker Diabetic Fatty rat; diabetic kidney disease; hydronephrosis; renal imaging; type 2 diabetes; μCT.
Conflict of interest statement
After the completion of this study, co-author Stinne Byrholdt Søgaard joined Novo Nordisk, Denmark, and co-author Iman Taghavi joined WSAudiology, Denmark. Neither company was involved in the design, execution, or funding of this study. This study’s funding body, the European Research Council, had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Nguyen I., van Koppen A., Joles J.A. Animal Models of Diabetic Kidney Disease. In: Roelofs J.J., Vogt L., editors. Diabetic Nephropathy: Pathophysiology and Clinical Aspects. Springer International Publishing; Cham, Switzerland: 2019. pp. 375–413. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

