MXene-Supported Single-Atom Electrocatalysts
- PMID: 40150844
- PMCID: PMC12061334
- DOI: 10.1002/advs.202414674
MXene-Supported Single-Atom Electrocatalysts
Abstract
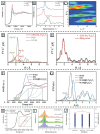
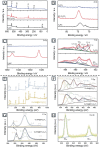
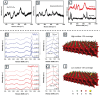
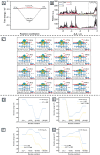
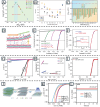
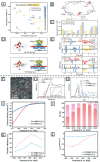
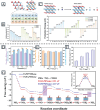
MXenes, a novel member of the 2D material family, shows promising potential in stabilizing isolated atoms and maximizing the atom utilization efficiency for catalytic applications. This review focuses on the role of MXenes as support for single-atom catalysts (SACs) for various electrochemical reactions, namely the hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), carbon dioxide reduction reaction (CO2RR), and nitrogen reduction reaction (NRR). First, state-of-the-art characterization and synthesis methods of MXenes and MXene-supported SACs are discussed, highlighting how the unique structure and tunable functional groups enhance the catalytic performance of pristine MXenes and contribute to stabilizing SAs. Then, recent studies of MXene-supported SACs in different electrocatalytic areas are examined, including experimental and theoretical studies. Finally, this review discusses the challenges and outlook of the utilization of MXene-supported SACs in the field of electrocatalysis.
Keywords: MXenes; catalytic applications; single‐atom catalysts; support; synthesis and characterization.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Hu E., Feng Y., Nai J., Zhao D., Hu Y., Lou X. W., Energ. Environ. Sci. 2018, 11, 872;
- b) You B., Sun Y., Acc. Chem. Res. 2018, 51, 1571. - PubMed
-
- Luo Y., Zhang Z., Chhowalla M., Liu B., Adv. Mater. 2022, 34, 2108133. - PubMed
-
- a) Dawood F., Anda M., Shafiullah G. M., Int. J. of Hydrogen Energ. 2020, 45, 3847;
- b) Wang Y.‐Z., Ding Y.‐M., Zhang C.‐H., Xue B.‐W., Li N.‐W., Yu L., Rare Met. 2021, 40, 2785;
- c) Jiang N., You B., Sheng M., Sun Y., Angew. Chem. Int. Ed. Engl. 2015, 54, 6251. - PubMed
-
- Mushtaq M. A., Kumar A., Liu W., Ji Q., Deng Y., Yasin G., Saad A., Raza W., Zhao J., Ajmal S., Wu Y., Ahmad M., Lashari N. U. R., Wang Y., Li T., Sun S., Zheng D., Luo Y., Cai X., Sun X., Adv. Mater. 2024, 36, 2313086. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
