Non-canonical functions of BCL-2 family members in energy metabolism and necrotic cell death regulation
- PMID: 40150937
- PMCID: PMC12296072
- DOI: 10.1080/15384101.2025.2484868
Non-canonical functions of BCL-2 family members in energy metabolism and necrotic cell death regulation
Abstract
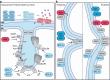
The large family of BCL-2 proteins plays a well-established role in the regulation of mitochondrial apoptosis pathway, and the crosstalk between death receptor signaling and mitochondrial apoptosis. Accumulating evidence suggests, however, that various BCL-2 family members are also involved in the regulation of apoptosis-unrelated necrotic forms of cell death, and even non-cell death processes. In this review, we discuss the emerging role of BCL-2 family members, and in particular BIM, in the regulation of mitochondrial dynamics, morphology and energy metabolism, and associated consequences for drug-inuced necrotic cell death.
Keywords: BCL-2; apoptosis; electron transport chain; glycolysis; mitochondria; mitotoxicants.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
