Integrating network pharmacology and experimental validation to uncover the synergistic effects of Huangqi ()-Ezhu () with 5-fluorouracil in colorectal cancer models
- PMID: 40151125
- PMCID: PMC11955770
- DOI: 10.19852/j.cnki.jtcm.2025.02.004
Integrating network pharmacology and experimental validation to uncover the synergistic effects of Huangqi ()-Ezhu () with 5-fluorouracil in colorectal cancer models
Abstract
Objective: To evaluate the effects of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) (HQEZ) on colorectal cancer therapies and to elucidate the potential mechanisms of HQEZ, especially in combination with 5-Fluorouracil (5-FU).
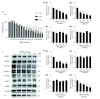
Methods: The anti-tumor effects of HQEZ were evaluated in colorectal cancer models both in vivo and in vitro. The network pharmacological assay was used to investigate potential mechanisms of HQEZ. Potential target genes were selected by Gene Ontology (GO) enrichment analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, protein-protein interaction network (PPI) and molecular docking. Within key targets, potential targets related to drug sensitivity, especially the sensitivity to 5-FU, were evaluated in HCT116 in vitro by immunofluorescence, quantitative real-time polymerase chain reaction (qPCR) and Western-blot. Then, changes in potential targets were assessed in tumors from tumor-bearing mice and the expression of these targets was also evaluated in colorectal cancer (COAD) patients from the Cancer Genome Atlas Program (TCGA) database.
Results: HQEZ significantly enhanced the anti-tumor activity of 5-FU in vivo and inhibit the growth of HCT116 in vitro. By network pharmacological analysis, key targets, such as protein kinase B (AKT1), epidermal growth factor receptor (EGFR), adenosine triphosphate (ATP) binding cassette subfamily B member 1 (ABCB1, also named multidrug resistance protein 1, MDR1), ATP binding cassette subfamily G member 2 (ABCG2), thymidylate synthetase (TYMS, also named TS), prostaglandin-endoperoxide synthase 2 (PTGS2), matrix metallopeptidase 2 (MMP2), MMP9, toll like receptor 4 (TLR4), TLR9 and dihydropyrimidine dehydrogenase (DPYD), were identified. Additionally, 4 potential core active ingredients (Folate, Curcumin, quercetin and kaempferol) were identified to be important for the treatment of colorectal cancer with HQEZ. In key targets, chemoresistance related targets were validated to be affected by HQEZ. Furthermore, 5-FU sensitivity related targets, including MDR1, TS, EGFR, ribonucleotide reductase catalytic subunit M1, Breast and Ovarian Cancer Susceptibility Protein 1 (BRCA1) and mutl homolog 1 were also significantly reduced by HQEZ both in vitro and in vivo. Finally, these validated key targets and 5-FU sensitivity related targets were demonstrated to be up-regulated in COAD patients based on TCGA database.
Conclusion: HQEZ has synergistic effects on the anti-tumor activity of 5-FU in the treatment of colorectal cancer both in vivo and in vitro. The beneficial effect of HQEZ results from the inhibition of the drug sensitivity targets associated with 5-FU. The combination therapy of HQEZ with 5-FU or other chemotherapeutic drugs will also improve the anti-tumor efficacy of chemotherapy.
Keywords: Huangqi ()-Ezhu (); colorectal neoplasms; fluorouracil; network pharmacology; parasitic sensitivity tests.
Figures



References
-
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet 2019; 394: 1467-80. - PubMed
MeSH terms
Substances
Grants and funding
- 82003961/National Natural Science Foundation of China Youth Found Project: Anti-Colorectal Cancer Metastasis Mechanism of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) According to the Hypoxia-Inducible Factor 2 Alpha/β-Catenin Cross-Talk Which Influence the Colon Tumor Stem Cells in Hypoxia Microenvironment
- 82104408/National Natural Science Foundation of China Youth Found Project: the Mechanism of the Compatibility of Huangqi (Radix Astragali Mongolici) and Ezhu (Rhizoma Curcumae Phaeocaulis) on the Early Metastasis of Hepatocellular Carcinoma Mediated by Cancer Associated Fibroblasts
- 82074035/Science Foundation of China Project: Involvement of Hypoxia Inducible Factor-1α Signal in Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) Combination Induced Remolding of Tumor Hypoxic Microenvironment
- YB201921/Science and Technology Development Project of Traditional Chinese Medicine in Jiangsu Province: Mechanism of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) Herb Pair on the Inhibition of Colon Cancer Metastasis Through the Wnt/β-catenin Pathway
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
