Exploring the Nuclearity and Structural Motifs of Phenoxyimine Alkaline Earth Complexes
- PMID: 40151377
- PMCID: PMC11938394
- DOI: 10.1021/acs.organomet.4c00504
Exploring the Nuclearity and Structural Motifs of Phenoxyimine Alkaline Earth Complexes
Abstract
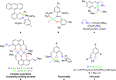
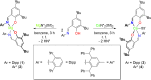
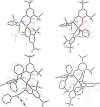
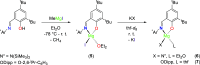
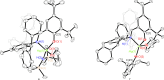
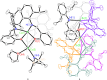
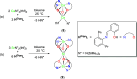
The nuclearity and structural motifs of alkaline earth complexes supported by bidentate phenoxyimine ligands has been explored by modulation of the stereoelectronic profile of the ligand, the atomic number of the metal, and the synthetic protocol. Changing the size of the N-imine substituents was found to have no effect on protonolysis reactions between [MgN″2]2 or CaN″2(thf)2 (N″ = N(SiMe3)2) and H t Bu2,ArL (1-OH-2-CH = NAr-4,6- t Bu-C6H2; Ar = 2,6-iPr-C6H3 = Dipp or 2,6-CHPh2-4-Me-C6H2 = Ar*) regardless of reaction stoichiometry, with homoleptic bis(ligand) complexes ( t Bu2,DippL)2Mg (1), ( t Bu2,Ar*L)2Mg (2), ( t Bu2,DippL)2Ca(thf) (3) and ( t Bu2,Ar*L)2Ca(thf) (4) isolated. The importance of reaction protocol was demonstrated by the facile isolation of heteroleptic complex ( t Bu2,Ar*L)MgI(OEt2) (5) from the reaction of equimolar amounts of H t Bu2,Ar*L and MeMgI. Importantly, no subsequent ligand redistribution was observed when complex 5 readily reacted with KN" or KODipp to form ( t Bu2,Ar*L)Mg{N(SiMe3)2}(OEt2) (6) and ( t Bu2,Ar*L)Mg(ODipp)(thf) (7). When small 4,6-phenoxide substituents were considered (HH2,DippL), multimetallic clusters were afforded: (H2,DippL)3Ca2(N″)(thf) (8) and (H2,DippL)6Sr3 (9).
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Hans Wedepohl K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59 (7), 1217–1232. 10.1016/0016-7037(95)00038-2. - DOI
- Hill M. S.; Liptrot D. J.; Weetman C. Alkaline earths as main group reagents in molecular catalysis. Chem. Soc. Rev. 2016, 45 (4), 972–988. 10.1039/C5CS00880H. - DOI - PubMed
- Harder S.; Langer J. Opportunities with calcium Grignard reagents and other heavy alkaline-earth organometallics. Nat. Rev. Chem. 2023, 7 (12), 843–853. 10.1038/s41570-023-00548-0. - DOI - PubMed
- Bhoelan B. S.; Stevering C. H.; van der Boog A. T.; van der Heyden M. A. Barium toxicity and the role of the potassium inward rectifier current. Clin. Toxicol. 2014, 52 (6), 584–593. 10.3109/15563650.2014.923903. - DOI - PubMed
-
- Schlenk W.; jun W. S. Über die Konstitution der Grignardschen Magnesiumverbindungen. Ber. Dtsch. Chem. Ges. A, B 1929, 62 (4), 920–924. 10.1002/cber.19290620422. - DOI
-
- Knüpfer C.; Langer J.; Harder S. bis-Silyl-triazenide ligands in alkaline-earth metal chemistry. Z. Anorg. Allg. Chem. 2024, 650 (3), e20230022610.1002/zaac.202300226. - DOI
- Stevens M. P.; Spray E.; Vitorica-Yrezabal I. J.; Singh K.; Timmermann V. M.; Sotorrios L.; Macgregor S. A.; Ortu F. Synthesis, characterisation and reactivity of group 2 complexes with a thiopyridyl scorpionate ligand. Dalton Trans. 2022, 51 (31), 11922–11936. 10.1039/D2DT02012B. - DOI - PubMed
- Barros M. L.; Cushion M. G.; Schwarz A. D.; Turner Z. R.; Mountford P. Magnesium, calcium and zinc [N2N′] heteroscorpionate complexes. Dalton Trans. 2019, 48 (13), 4124–4138. 10.1039/C8DT04985H. - DOI - PubMed
- Chapple P. M.; Kahlal S.; Cartron J.; Roisnel T.; Dorcet V.; Cordier M.; Saillard J.-Y.; Carpentier J.-F.; Sarazin Y. Bis(imino)carbazolate: A Master Key for Barium Chemistry. Angew. Chem., Int. Ed. 2020, 59 (23), 9120–9126. 10.1002/anie.202001439. - DOI - PubMed
- Gentner T. X.; Rösch B.; Ballmann G.; Langer J.; Elsen H.; Harder S. Low Valent Magnesium Chemistry with a Super Bulky β-Diketiminate Ligand. Angew. Chem., Int. Ed. 2019, 58 (2), 607–611. 10.1002/anie.201812051. - DOI - PubMed
- Liu Y.; Zhu K.; Chen L.; Liu S.; Ren W. Azobenzenyl Calcium Complex: Synthesis and Reactivity Studies of a Ca(I) Synthon. Inorg. Chem. 2022, 61 (50), 20373–20384. 10.1021/acs.inorgchem.2c03008. - DOI - PubMed
- Yang W.; White A. J. P.; Crimmin M. R. Deoxygenative Coupling of CO with a Tetrametallic Magnesium Hydride Complex. Angew. Chem., Int. Ed. 2024, 63 (14), e20231962610.1002/anie.202319626. - DOI - PMC - PubMed
- Piesik D. F. J.; Range S.; Harder S. Bimetallic Calcium and Zinc Complexes with Bridged β-Diketiminate Ligands: Investigations on Epoxide/CO2 Copolymerization. Organometallics 2008, 27 (23), 6178–6187. 10.1021/om800597f. - DOI
-
- Gärtner M.; Görls H.; Westerhausen M. Synthesis and structural variations of substituted phenylamide complexes of the heavy alkaline earth metals calcium, strontium and barium. Dalton Trans. 2008, (12), 1574–1582. 10.1039/b717267b. - DOI - PubMed
- Boyle T. J.; Sears J. M.; Greathouse J. A.; Perales D.; Cramer R.; Staples O.; Rheingold A. L.; Coker E. N.; Roper T. M.; Kemp R. A. Synthesis and Characterization of Structurally Diverse Alkaline-Earth Salen Compounds for Subterranean Fluid Flow Tracking. Inorg. Chem. 2018, 57 (5), 2402–2415. 10.1021/acs.inorgchem.7b01350. - DOI - PubMed
-
- Santoro O.; Zhang X.; Redshaw C. Synthesis of Biodegradable Polymers: A Review on the Use of Schiff-Base Metal Complexes as Catalysts for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2020, 10 (7), 80010.3390/catal10070800. - DOI
- Juyal V. K.; Pathak A.; Panwar M.; Thakuri S. C.; Prakash O.; Agrwal A.; Nand V. Schiff base metal complexes as a versatile catalyst: A review. J. Organomet. Chem. 2023, 999, 12282510.1016/j.jorganchem.2023.122825. - DOI
- Al Zoubi W.; Ko Y. G. Organometallic complexes of Schiff bases: Recent progress in oxidation catalysis. J. Organomet. Chem. 2016, 822, 173–188. 10.1016/j.jorganchem.2016.08.023. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
