EUS-guided lauromacrogol ablation with different concentrations of lauromacrogol for the treatment of pancreatic cystic neoplasm: A randomized controlled study
- PMID: 40151595
- PMCID: PMC11939937
- DOI: 10.1097/eus.0000000000000105
EUS-guided lauromacrogol ablation with different concentrations of lauromacrogol for the treatment of pancreatic cystic neoplasm: A randomized controlled study
Abstract
Objectives: To explore the safety and efficacy of injections of 1%, 2%, or 3% lauromacrogol during EUS-guided lauromacrogol ablation (EUS-LA) for the treatment of pancreatic cystic neoplasms (PCNs) and to determine the optimal concentration of lauromacrogol for use in EUS-LA therapeutic regimens.
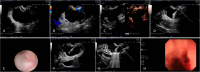
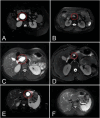
Methods: From May 2021 to January 2023, patients who met the indications for EUS-LA were randomly divided into 3 groups: A, B, and C; the patients in these groups were injected with 1%, 2%, and 3% lauromacrogol during EUS-LA, respectively. Safety was evaluated based on the incidence of postoperative complications. Efficacy was comprehensively evaluated by assessing the ablation rate and ablation effect.
Results: Forty-two patients underwent EUS-LA, and 31 patients completed at least 1 postoperative re-examination. No acute pancreatitis was observed in the 1% and 2% lauromacrogol groups, and 1 case of acute pancreatitis occurred in the 3% lauromacrogol group. The total complication rate was 2.4%. The median ablation rates of the groups were 94.1%, 82.0%, and 100.0%, respectively. There were statistically significant differences in the EUS-LA ablation rate between the 1% and 3% lauromacrogol groups and between the 2% and 3% lauromacrogol groups. There was a statistically significant difference in complete disappearance between the 1% and 3% lauromacrogol groups as well as between the 2% and 3% lauromacrogol groups.
Conclusion: The short-term outcomes showed that injections of 1%, 2%, and 3% lauromacrogol were safe for use in EUS-LA, and injection of 3% lauromacrogol was the most effective for EUS-LA.
Keywords: Ablation; EUS; Lauromacrogol; Pancreatic cystic neoplasm.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc on behalf of Scholar Media Publishing.
Conflict of interest statement
Enqiang Linghu is an Associate Editor of the journal. The article was subjected to the standard procedures of the journal, with a review process independent of the editor and his research group.
Figures


References
-
- de Jong K Nio CY Hermans JJ, et al. . High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806–811. - PubMed
-
- Girometti R Intini S Brondani G, et al. . Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging 2011;36:196–205. - PubMed
-
- Ip IK, Mortele KJ, Prevedello LM, Khorasani R. Focal cystic pancreatic lesions: assessing variation in radiologists' management recommendations. Radiology 2011;259:136–141. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

