Multimodal monitoring of neutrophil activity during cardiac surgery
- PMID: 40151619
- PMCID: PMC11947689
- DOI: 10.3389/fimmu.2025.1504944
Multimodal monitoring of neutrophil activity during cardiac surgery
Abstract
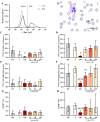
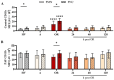
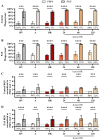
Cardiac surgery and the associated ischemia-reperfusion injury trigger an inflammatory response, which, in turn, can contribute to organ damage, prolonged hospitalization, and mortality. Therefore, the present study performed comprehensive monitoring of neutrophil-related inflammation in patients who underwent aortic valve surgery, including extracorporeal circulation. Neutrophil-related inflammation, as well as alterations in cellular physiology, phenotype, and function, were analyzed by flow cytometry, ELISA, and microscopy. Neutrophil activation occurred intraoperatively and preceded the upregulation of conventional inflammatory markers such as C-reactive protein and interleukin-6. Perioperatively, neutrophils maintained a stable response to platelet-activating factor (PAF) with regard to CD11b and CD66b expression but showed a decreased response in CD10. Postoperatively, neutrophils exhibited marked alterations in PAF-induced depolarization, while reactive oxygen species generation and phagocytic activity remained largely stable. Surprisingly, platelet-neutrophil complex formation was severely impaired intraoperatively but returned to normal levels postoperatively. Further studies are needed to elucidate the implications of these intraoperative and postoperative changes in neutrophil and platelet activity with respect to a potential immune dysfunction that temporarily increases susceptibility to infectious or hemostatic complications.
Keywords: cardiac surgery; inflammation; ischemia-reperfusion injury; neutrophil granulocytes; platelet-activating factor; platelet-neutrophil complexes; platelets; thrombocytes.
Copyright © 2025 Jovanovski, Wohlgemuth, Lessing, Hüsken, Koller, Thomaß, Müller, Mannes, Nungeß, Jovanovska, Mühling, Liebold, Huber-Lang and Messerer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

