Transforming Growth Factor Beta2 Promotes Migration and Inhibits the Proliferation of Gastric Cancer Cells by Regulating the pSmad2/3-NDRG1 Signaling Pathway
- PMID: 40151835
- PMCID: PMC11949502
- DOI: 10.1002/mco2.70148
Transforming Growth Factor Beta2 Promotes Migration and Inhibits the Proliferation of Gastric Cancer Cells by Regulating the pSmad2/3-NDRG1 Signaling Pathway
Abstract
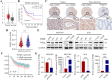
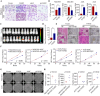
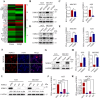
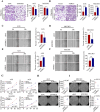
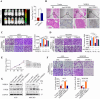
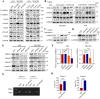
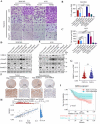
Transforming growth factor beta2 (TGFβ2) is upregulated in gastric cancer (GC), playing a crucial role in driving its progression. However, the biological effects of TGFβ2 in GC metastasis and proliferation remain not fully understood. Our study reveals that TGFβ2 enhances N-myc downstream-regulated gene 1 (NDRG1) protein expression by activating the TGFβR/Smad2/3-dependent pathway, accelerating GC progression. TGFβ2 knockdown downregulates NDRG1 by inhibiting the TGFβR/Smad2/3 signaling pathway, which in turn inhibits GC cell migration and epithelial-mesenchymal transition (EMT) but stimulates proliferation. Both TGFβ2 upregulation and NDRG1 upregulation enhance GC cell migration in vitro and promote lung metastasis in mouse models. Interfering with NDRG1 reverses TGFβ2-induced migration, and inhibiting Smad2/3 or TGFβR reverses TGFβ2-induced NDRG1 upregulation and GC cell migration. Clinical sample analysis shows high TGFβ2 and NDRG1 expression in GC, associated with poor prognosis. Our study reveals that TGFβ2 upregulates NDRG1 via the TGFβR/Smad2/3 pathway, driving GC progression and highlighting the potential role of the TGFβ2NDRG1 axis in GC-targeted therapies.
Keywords: N‐myc downstream‐regulated gene 1; gastric cancer; metastasis; proliferation; transforming growth factor beta2.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71, no. 3 (2021): 209–249. - PubMed
-
- Chinese Gastric Cancer Association of Chinese Anti‐Cancer Association , Chinese Society of Upper Gastrointestinal Surgeons of Chinese Medical Doctor Association , and Chinese Health Risk Management Collaboration‐Gastric Cancer Group , “[Chinese Guideline on Risk Management of Gastric Cancer in the General Public(2023 Edition)],” Zhonghua Yi Xue Za Zhi 103, no. 36 (2023): 2837–2849. - PubMed
-
- Li C., Zheng Y., Shi Z., et al. “Perioperative Camrelizumab (C) Combined With Rivoceranib (R) and Chemotherapy (Chemo) Versus Chemo for Locally Advanced Resectable Gastric or Gastroesophageal Junction (G/GEJ) Adenocarcinoma: The First Interim Analysis of a Randomized, Phase III Trial (DRAGON IV),” Annals of Oncology 34, no. supplement S2 (2023): S852–S886.
-
- Allemani C., Matsuda T., Di Carlo V., et al., “Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD‐3): Analysis of Individual Records for 37 513 025 Patients Diagnosed With One of 18 Cancers From 322 Population‐Based Registries in 71 Countries,” Lancet 391, no. 10125 (2018): 1023–1075. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
