Quantitative Analysis of 3D Anatomy to Inform Planning of Ductal Arteriosus Stenting
- PMID: 40152008
- PMCID: PMC12162213
- DOI: 10.1002/ccd.31510
Quantitative Analysis of 3D Anatomy to Inform Planning of Ductal Arteriosus Stenting
Abstract
Background: Ductus arteriosus stenting (DAS) is used to palliate infants with ductal-dependent pulmonary blood flow (DD-PBF), however patent ductus arteriosus (PDA) anatomy can be complex and heterogenous.
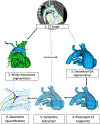
Aims: We developed custom, open-source software to model and quantify PDA anatomy.
Methods: We retrospectively identified 33 neonates with DD-PBF with a CTA before DAS. A novel custom workflow was implemented in 3D Slicer and SlicerHeart to semi-automatically extract centerlines of the course of the PDA and surrounding vessels. 3D ductal length, diameter, curvature and tortuosity were automatically calculated (3D automatic) and compared to manually adjusted 3D measurements (3D semi-automatic), and manual measurements of PDA dimensions in 2D projectional angiograms before and after stent angioplasty.
Results: Ductal anatomy was successfully modeled and quantified in all subjects. 3D automatic and semi-automatic measurements of straight-line aortic to pulmonary artery length were not significantly different than 2D measurements. Semi-automatic 3D measurements were similar to 2D measurements of the total length. Minimum and maximum ductal diameters were not significantly different by 3D automatic and 2D measurements, however semi-automatic 3D diameters were significantly larger. Inter-reader reliability of ductal length and diameter was higher with manual adjustment of 3D centerlines compared to standard measurement of 2D angiograms. These differences were consistent across PGE doses between CTA and DAS.
Conclusions: Automatic PDA modeling is feasible and efficient, enabling reproducible quantification of ductal anatomy for procedural planning of DAS in patients with DD-PBF. Further development is needed as well as investigation of whether 3D modeling-derived measurements influence procedural duration or outcome.
Keywords: 3D modeling; CT angiography; PDA stent; congenital heart disease; quantification.
© 2025 Wiley Periodicals LLC.
Conflict of interest statement
Conflicts of Interest Statement
No relevant conflicts of interest to disclose.
Figures

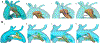
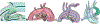


Similar articles
-
Preprocedural three-dimensional planning aids in transcatheter ductal stent placement: A single-center experience.Catheter Cardiovasc Interv. 2020 May 1;95(6):1141-1148. doi: 10.1002/ccd.28669. Epub 2019 Dec 18. Catheter Cardiovasc Interv. 2020. PMID: 31854085 Free PMC article.
-
Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting.Catheter Cardiovasc Interv. 2019 Apr 1;93(5):933-943. doi: 10.1002/ccd.28125. Epub 2019 Feb 21. Catheter Cardiovasc Interv. 2019. PMID: 30790426
-
Use of carotid and axillary artery approach for stenting the patent ductus arteriosus in infants with ductal-dependent pulmonary blood flow: A multicenter study from the congenital catheterization research collaborative.Catheter Cardiovasc Interv. 2020 Mar 1;95(4):726-733. doi: 10.1002/ccd.28631. Epub 2019 Dec 9. Catheter Cardiovasc Interv. 2020. PMID: 31815357
-
Advances in Pediatric Ductal Intervention: an Open or Shut Case?Curr Cardiol Rep. 2020 Jan 29;22(3):14. doi: 10.1007/s11886-020-1266-x. Curr Cardiol Rep. 2020. PMID: 31997085 Review.
-
Stenting of the ductus arteriosus for ductal-dependent pulmonary blood flow-current techniques and procedural considerations.Congenit Heart Dis. 2019 Jan;14(1):110-115. doi: 10.1111/chd.12709. Congenit Heart Dis. 2019. PMID: 30811792 Review.
References
-
- Glatz AC, Petit CJ, Goldstein BH, et al. Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants With Ductal-Dependent Pulmonary Blood Flow: Insights From the Congenital Catheterization Research Collaborative. Circulation 2018;137(6):589–601. DOI: 10.1161/CIRCULATIONAHA.117.029987. - DOI - PubMed
-
- Bentham JR, Zava NK, Harrison WJ, et al. Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates With Duct-Dependent Pulmonary Blood Flow: Associations With Clinical Outcomes in a Multicenter National Study. Circulation 2018;137(6):581–588. DOI: 10.1161/CIRCULATIONAHA.117.028972. - DOI - PubMed
-
- Lemley BA, Wu L, Roberts AL, et al. Trends in Ductus Arteriosus Stent Versus Blalock-Taussig-Thomas Shunt Use and Comparison of Cost, Length of Stay, and Short-Term Outcomes in Neonates With Ductal-Dependent Pulmonary Blood Flow: An Observational Study Using the Pediatric Health Information Systems Database. J Am Heart Assoc 2023;12(23):e030575. DOI: 10.1161/JAHA.123.030575. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- R01 HL153166/HL/NHLBI NIH HHS/United States
- This study was supported by NIH R01HL153166, an Additional Ventures Expansion Award, Additional Ventures Single Ventricle Research Fund, The Topolewski Endowed Chair at the Children's Hospital of Philadelphia, T32 HL0097915, CHOP Cardiac Center Research Grant.
LinkOut - more resources
Full Text Sources

