Chemical ecology of symbioses in cycads, an ancient plant lineage
- PMID: 40152178
- PMCID: PMC12018785
- DOI: 10.1111/nph.70109
Chemical ecology of symbioses in cycads, an ancient plant lineage
Abstract
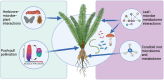
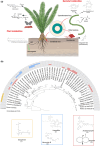
Cycads are an ancient lineage of gymnosperms that maintain a plethora of symbiotic associations from across the tree of life. They have myriad morphological, structural, physiological, chemical, and behavioral adaptations that position them as a unique system to study the evolution, ecology, and mechanism of symbiosis. To this end, we have provided an overview of cycad symbiosis biology covering insects, bacteria, and fungi, and discuss the most recent advances in the underlying chemical ecology of these associations.
Las cícadas son un antiguo linaje de gimnospermas que contienen una plétora de asociaciones simbióticas con organismos de todo el árbol de la vida. Ellas presentan una miríada de adaptaciones morfológicas, estructurales, fisiológicas, químicas y de comportamiento que las sitúan como un sistema único para estudiar la evolución, la ecología y el mecanismo de simbiosis. Con este fin, hemos proporcionado una visión general de la biología de la simbiosis de las cícadas que abarca insectos, bacterias y hongos, y discutimos los avances más recientes en la ecología química relacionada a estas asociaciones.
Keywords: chemical ecology; cyanobacteria; cycads; gymnosperms; microbiome metabolites; plant secondary metabolites; symbiosis; volatile organic compounds.
© 2025 The Author(s). New Phytologist © 2025 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures


References
-
- Bell‐Doyon P, Laroche J, Saltonstall K, Villlarreal JC. 2020. Specialized bacteriome uncovered in the coralloid roots of the epiphytic gymnosperm, Zamia pseudoparasitica . Environmental DNA 2: 418–428.
-
- Bellenger JP, Darnajoux R, Zhang X, Kraepiel AML. 2020. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: a review. Biogeochemistry 149: 53–73.
-
- Bellenger JP, Xu Y, Zhang X, Morel FMM, Kraepiel AML. 2014. Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2‐fixing bacteria in soils. Soil Biology and Biochemistry 69: 413–420.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

