Criteria to Report Adverse Drug Withdrawal Events in Clinical Trials: A Systematic Review
- PMID: 40152210
- PMCID: PMC12205270
- DOI: 10.1111/jgs.19457
Criteria to Report Adverse Drug Withdrawal Events in Clinical Trials: A Systematic Review
Abstract
Background: Polypharmacy is a major risk factor for adverse drug events (ADEs), which are a common cause of hospitalization, especially among older adults. Deprescribing is a promising strategy to prevent ADEs; however, clinicians may hesitate to deprescribe for fear of causing adverse drug withdrawal events (ADWEs). Collectively, ADWEs are the re-emergence of symptoms or a disease state due to the discontinuation of a medication. Although capturing ADWEs is critical to understanding the complications that might arise from deprescribing, these events may not be routinely or systematically captured in clinical trials.
Objectives: We aimed to determine the frequency of ADWE reporting, compare the strengths and limitations of different approaches, and compare the rates of the number of ADWEs detected across trials.
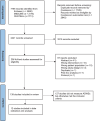
Methods: A systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. The search strategy was developed with a research librarian, and studies were identified using Ovid Medline, Embase, and the Cochrane Central Register of Controlled Trials from inception to July 2, 2024. We included all randomized controlled trials testing a deprescribing intervention in older adults (mean or median age ≥ 65 years) and analyzed a subsample of the studies reporting ADWEs as an outcome.
Results: Among the 139 eligible studies that were identified, only 12 reported an ADWE. These studies utilized 6 approaches to capture ADWEs: Naranjo ADWE Probability Scale; clinical monitoring for specific withdrawal symptoms; identification through ICD-10 codes; identification of ADWEs as a subset of confirmed ADEs; patient/caregiver self-report; and clinical judgment.
Conclusion: Results confirmed that few deprescribing studies capture ADWEs and there is a lack of standardized reporting. A harmonized approach to capturing ADWEs with specific criteria could ensure more consistent results in deprescribing trials, improve our understanding of this important outcome, and facilitate future meta-analyses.
Keywords: adverse drug events; adverse drug withdrawal events; clinical trials; deprescribing; polypharmacy.
© 2025 The Author(s). Journal of the American Geriatrics Society published by Wiley Periodicals LLC on behalf of The American Geriatrics Society.
Conflict of interest statement
Emily G. McDonald and Todd C. Lee hold the copyright for the MedSafer software in conjunction with McGill University.
Figures

References
-
- Coulson J. and Routledge P. A., “Adverse Reactions to Drug Withdrawal,” Adverse Drug Reaction Bulletin 252, no. 252 (2008): 967–970, 10.1097/fad.0b013e328323a63b. - DOI
-
- Dahal R. and Bista S., “Strategies to Reduce Polypharmacy in the Elderly,” PubMed, 2024, https://www.ncbi.nlm.nih.gov/books/NBK574550.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

