Quantitative performance of humanized plasma and epithelial lining fluid exposures of meropenem, cefiderocol and tobramycin against a challenge set of Klebsiella pneumoniae and Pseudomonas aeruginosa in a standardized neutropenic murine pneumonia model
- PMID: 40152266
- PMCID: PMC12129580
- DOI: 10.1093/jac/dkaf100
Quantitative performance of humanized plasma and epithelial lining fluid exposures of meropenem, cefiderocol and tobramycin against a challenge set of Klebsiella pneumoniae and Pseudomonas aeruginosa in a standardized neutropenic murine pneumonia model
Abstract
Background: The COMBINE murine neutropenic pneumonia model looks to standardize an important element of preclinical development and provide interlaboratory uniformity. Herein we provide quantitative bacterial density in lung benchmark efficacy data of humanized exposures of meropenem, cefiderocol and tobramycin in plasma and epithelial lining fluid (ELF) against a collection of Klebsiella pneumoniae and Pseudomonas aeruginosa.
Methods: In accordance with the COMBINE protocol, human-simulated regimens (HSRs) based on both plasma and ELF exposures of meropenem, cefiderocol (both as 2 g q8h as 3 h infusions) and tobramycin 7 mg/kg as 30 min infusions were tested against K. pneumoniae and P. aeruginosa isolates. The 24 h change in cfu/lung for each HSR was calculated. Each isolate was tested in duplicate against both the plasma and ELF HSRs on separate experiment days.
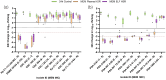
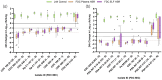
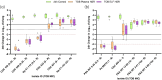
Results: Meropenem HSRs demonstrated >1 log10 kill against all P. aeruginosa isolates with MICs of ≤16 mg/L, but only against K. pneumoniae isolates with MICs of ≤2 mg/L as isolates with MICs of >2 mg/L generally harboured carbapenemases. Cefiderocol HSRs uniformly achieved >1 log10 kill against both species at MICs of ≤8 mg/L, with net growth and extensive variability in P. aeruginosa isolates with MICs of 16 mg/L. All tobramycin-susceptible isolates demonstrated >1 log10 kill, while non-susceptible isolates did not. Differences in cfu/lung magnitude between the plasma and ELF HSRs were most pronounced around the clinical breakpoints.
Conclusions: In the COMBINE pneumonia model, administration of plasma and ELF HSRs of meropenem, cefiderocol and tobramycin demonstrated 24 h cfu/lung within reason of expectation given known PK/PD properties and existing clinical breakpoints.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
Similar articles
-
Quantitative performance of humanized serum and epithelial lining fluid exposures of tigecycline and levofloxacin against a challenge set of Klebsiella pneumoniae and Pseudomonas aeruginosa in a standardized neutropenic murine pneumonia model.J Antimicrob Chemother. 2024 Dec 2;79(12):3142-3149. doi: 10.1093/jac/dkae333. J Antimicrob Chemother. 2024. PMID: 39423017 Free PMC article.
-
Development and confirmation of humanized plasma and epithelial lining fluid exposures of meropenem, cefiderocol and tobramycin in a standardized neutropenic murine pneumonia model.J Antimicrob Chemother. 2025 Feb 3;80(2):478-484. doi: 10.1093/jac/dkae432. J Antimicrob Chemother. 2025. PMID: 39657022 Free PMC article.
-
Comparison of the in vivo efficacy and resistance development potential between cefiderocol and ceftolozane/tazobactam human simulated exposures against Pseudomonas aeruginosa in 72-hour murine thigh infection model.Infect Dis (Lond). 2025 Jul;57(7):658-668. doi: 10.1080/23744235.2025.2471822. Epub 2025 Mar 5. Infect Dis (Lond). 2025. PMID: 40042366
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
A Systematic Review of the Pharmacokinetics and Pharmacodynamics of Novel Beta-Lactams and Beta-Lactam with Beta-Lactamase Inhibitor Combinations for the Treatment of Pneumonia Caused by Carbapenem-Resistant Gram-Negative Bacteria.Int J Antimicrob Agents. 2024 Sep;64(3):107266. doi: 10.1016/j.ijantimicag.2024.107266. Epub 2024 Jul 5. Int J Antimicrob Agents. 2024. PMID: 38971203
References
MeSH terms
Substances
Grants and funding
- Global Antimicrobial Resistance Innovation Fund
- UK Department of Health and Social Care
- Administration for Strategic Preparedness and Response
- U.S. Department of Health and Human Services
- WT224842/Wellcome
- WT_/Wellcome Trust/United Kingdom
- 27302C0028/ES/NIEHS NIH HHS/United States
- 75A50122C00028/Biomedical Advanced Research and Development Authority
- Bill & Melinda Gates Foundation
- Germany's Federal Ministry of Education and Research
- CARB-X
- Public Health Agency of Canada
- Novo Nordisk Foundation
- NH/NIH HHS/United States
- U.S. National Institute of Allergy and Infectious Diseases
- NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

