Ramucirumab and paclitaxel in second or greater lines of therapy in patients with HER2-positive gastroesophageal cancer: a single center study
- PMID: 40152313
- PMCID: PMC11950916
- DOI: 10.1093/oncolo/oyaf037
Ramucirumab and paclitaxel in second or greater lines of therapy in patients with HER2-positive gastroesophageal cancer: a single center study
Abstract
Background: Human epidermal growth factor receptor 2 (HER2) overexpression is present in approximately 20-25 of patients with advanced gastroesophageal adenocarcinoma (GEA). Upon progression on 1st line therapy, ramucirumab and paclitaxel (rampac) is given in ≥2 line setting regardless of HER2 status. We aim to assess whether ramucirumab is associated with better survival in HER2 positive(+) pts compared to those with HER2(-) disease.
Methods: We reviewed all consecutive adult patients with metastatic/unresectable GEA who were treated with rampac for ≥2nd line therapy at Princess Margaret Cancer Centre from 2010 to 2021. Progression free survival (PFS) and overall survival (OS) were defined as time from starting rampac to progression or death and estimated using the Kaplan-Meier method.
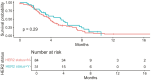
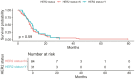
Results: There were 126 patients who received rampac following progression of 1st line chemotherapy, 96(76%) were male. The age at time of presentation and starting rampac was 59.0 ± 10.3 years and 59.9 ± 10.3 years, respectively. At the time of diagnosis, 32(25%) patients were HER2+. The majority of patients (n = 99;78%) received rampac in the 2L line setting compared to 28(22%) patients who received it in the 3rd/4th line setting. The median PFS and OS for HER2 + pts were 3.6 months and 9.4 months, respectively, which were similar to HER2- patients (median PFS = 3.6 months; median OS = 8.2 months). There was no statistically significant association between HER2 positivity and PFS (adjusted hazards ratio (HR) = 0.76, 95% confidence interval (CI) 0.48-1.22, P = .26), nor OS (adjusted HR = 0.88, 95% CI, 0.55-1.41, P = .59).
Conclusion: Rampac remains a valid treatment option for patients who are unable to participate in trials or do not have access to further HER2-directed therapy beyond first line.
Keywords: HER2; clinical oncology; esophageal cancer; gastric cancer; genes.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
LXM: Consulting—Eisai, Bristol Myers Squibb; RWJJ: Research Funding: AstraZeneca, Merck, Camurus Honoraria: BMS; EE: Consultant for: BMS, Zymeworks, Adaptimmune, Beigene, Jazz, Astellas, Virecta Tx, Natera, Abbvie, Daiichi –Sankyo, Roche Grant/Research support from: BMS, Zymeworks, Astra Zeneca, Jazz, Amgen, Bold Therapeutics, Arcus Biosciences Steering Committee Member: Jazz, Astra Zeneca. Additional financial relationship disclosures: Employment for: Merck (family member).
Figures
References
-
- Hofmann M, Stoss O, Shi D, et al.Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. https://doi.org/10.1111/j.1365-2559.2008.03028.x - DOI - PubMed
-
- Gravalos C, Jimeno A.. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. https://doi.org/10.1093/annonc/mdn169 - DOI - PubMed
-
- Tanner M, Hollmén M, Junttila TT, et al.Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. https://doi.org/10.1093/annonc/mdi064 - DOI - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al.; ToGA Trial Investigators. ToGA Trial Investigators: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. https://doi.org/10.1016/S0140-6736(10)61121-X - DOI - PubMed
-
- Van Cutsem E, Bang YJ, Feng-Yi F, et al.HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. https://doi.org/10.1007/s10120-014-0402-y - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous