Development and optimization of human T-cell leukemia virus-specific antibody-dependent cell-mediated cytotoxicity (ADCC) assay directed to the envelope protein
- PMID: 40152593
- PMCID: PMC12090781
- DOI: 10.1128/jvi.02268-24
Development and optimization of human T-cell leukemia virus-specific antibody-dependent cell-mediated cytotoxicity (ADCC) assay directed to the envelope protein
Abstract
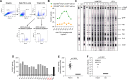
An estimated 10-20 million people worldwide are infected with the deltaretrovirus human T-cell leukemia virus type 1 (HTLV-1). Although most infected individuals remain asymptomatic, some progress to develop the fatal and debilitating disease adult T-cell leukemia/lymphoma (ATLL) or HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) or develop a plethora of other inflammatory disorders. In addition, HTLV-1 infection is associated with immunosuppression and a shorter lifespan. Although a protective role for neutralizing antibodies has been suggested, the role of non-neutralizing antibody-dependent cell-mediated cytotoxicity (ADCC) remains unclear, largely because an assay to measure this response has not been established. Here, we developed a high-throughput flow cytometry-based assay system to measure HTLV-1 envelope-specific ADCC. We used a natural killer cell-resistant T-lymphoblastoid cell line stably expressing the green fluorescent protein GFP to construct a target cell line expressing HTLV-1 envelope protein and using monoclonal antibodies and plasma samples from HTLV-infected or uninfected individuals, validating the specificity and sensitivity of the assay. We detected high ADCC activity in samples from HTLV-1-infected humans. In the plasma of experimentally infected macaques, ADCC activity was measured and a correlation between ADCC activity and HTLV-1 envelope antibody titers was observed. Further, we observed a significant increase in ADCC titer over time; as HTLV-1 infection persists, a higher ADCC response is generated, potentially influencing disease outcome. ADCC titer in HTLV-1-infected macaques also positively correlated with FLT3LG, IL-17F, CD4+ T cells, and lymphocytes but negatively correlated with monocyte frequency and classical monocyte frequency. In conclusion, these findings detail the generation of a cell line that enabled development of an HTLV-specific ADCC assay, which can be employed in large clinical studies as well as research involving humans or non-human primates.IMPORTANCEThis approach measures human T-cell leukemia virus (HTLV)-specific envelope antibody-dependent cell-mediated cytotoxicity responses, provides a critical tool to investigate the role of envelope-specific binding antibodies in the immune control of HTLV infection and pathogenesis, and may help guide the development of both therapeutic and preventative vaccine approaches.
Keywords: ADCC; CD16; Fc receptors; HTLV-1; adaptive immune response; natural killer cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

