Comparison Between MAIA and MP-3 In Healthy Subjects and Patients With Diabetes, Diabetic Retinopathy, and Age-Related Macular Degeneration
- PMID: 40152761
- PMCID: PMC11954536
- DOI: 10.1167/iovs.66.3.59
Comparison Between MAIA and MP-3 In Healthy Subjects and Patients With Diabetes, Diabetic Retinopathy, and Age-Related Macular Degeneration
Abstract
Purpose: The purpose of this study was to assess the comparability of mean sensitivity (MS) values obtained using the Macular Integrity Assessment (MAIA; CenterVue S.p.A. [iCare], Padova, Italy) and Microperimeter-3 (MP-3; NIDEK CO., Ltd., Gamagori, Japan) microperimetry (MP) devices.
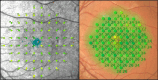
Methods: This cross-sectional study includes subjects with healthy eyes, eyes with diabetes mellitus without diabetic retinopathy (DM no DR), diabetic retinopathy (DR), and non-exudative age-related macular degeneration (AMD). Patients underwent testing on both MAIA and MP-3 MP devices, using a 10-2 macular grid with 68 stimuli and identical parameters. A diagnosis-adjusted linear regression model and mixed modeling mapped MP-3 MS to MAIA MS and Bland-Altman analysis were used to assess the agreement.
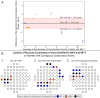
Results: One hundred eleven eyes (43 healthy eyes, 14 eyes with DM no DR, 32 eyes with DR, and 22 eyes with AMD) from 80 subjects (age = 57.2 ± 20.3 years) were enrolled. MAIA consistently showed higher MS than MP-3. The MP-3 device detected absolute scotomatous points in more eyes than MAIA (6 eyes [MAIA] vs. 10 eyes [MP-3]). Healthy eyes exhibited stronger agreements than those with DR (P < 0.001) or AMD (P = 0.015). Converting MP-3 to MAIA MS improved agreement and reduced coefficients of repeatability (CoRs) but did not fully account for inter-device variability. MP-3 classified more eyes as having relatively unstable or unstable fixation than MAIA (P = 0.014).
Conclusions: Both devices effectively detect retinal functional changes and scotomas. The conversion methods developed in this study can aid cross-device comparisons, but retinal pathologies (DM and AMD) introduce additional inter-device variability. Future studies incorporating multiple devices should account for this variability in their study design.
Conflict of interest statement
Disclosure:
Figures

References
-
- Pfau M, Jolly JK, Wu Z, et al. .. Fundus-controlled perimetry (microperimetry): application as outcome measure in clinical trials. Prog Retin Eye Res. 2021; 82: 100907. - PubMed
-
- Rohrschneider K, Bültmann S, Springer C.. Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog Retin Eye Res. 2008; 27(5): 536–548. - PubMed
-
- Heier JS, Pieramici D, Chakravarthy U, et al. .. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri Phase 3 trials. Ophthalmol Retina. 2020; 4(7): 673–688. - PubMed
-
- Balasubramanian S, Uji A, Lei J, Velaga S, Nittala M, Sadda SV.. Interdevice comparison of retinal sensitivity assessments in a healthy population: the CenterVue MAIA and the Nidek MP-3 microperimeters. Br J Ophthalmol. 2018; 102(1): 109–113. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

