Practical Anemia Bundle and Hemoglobin Recovery in Critical Illness: A Randomized Clinical Trial
- PMID: 40152861
- PMCID: PMC11953759
- DOI: 10.1001/jamanetworkopen.2025.2353
Practical Anemia Bundle and Hemoglobin Recovery in Critical Illness: A Randomized Clinical Trial
Abstract
Importance: Anemia is a common complication of surgery and acute illness that is associated with adverse clinical outcomes. The role of anemia prevention and treatment strategies in this setting remains unclear.
Objective: To evaluate the effect of a multifaceted anemia management bundle vs standard care on posthospitalization hemoglobin recovery and multidimensional functional outcomes in survivors of acute illness.
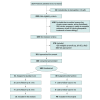
Design, setting, and participants: This parallel group randomized clinical trial, known as the Practical Anemia Bundle for Sustained Blood Recovery, was conducted at postsurgical and medical intensive care units at a large US medical center. Critically ill patients (aged ≥18 years) with moderate to severe anemia (hemoglobin concentration <10 g/dL) were enrolled between March 2022 and November 2023. Participants were randomly assigned 1:1 to the intervention or standard care group. Intention-to-treat analyses were performed between July 2024 and January 2025.
Intervention: The intervention bundle was delivered throughout the duration of hospitalization and included optimized phlebotomy practices, clinical decision support, and pharmacological anemia treatment with intravenous iron.
Main outcomes and measures: The primary outcome was the mean difference in hemoglobin concentration at 1 month after hospital discharge.
Results: A total of 100 patients (median [IQR] age, 68 [61-72] years; 57 men [57.0%]; 65 [65.0%] with postsurgical admission to the intensive care unit) were enrolled during acute illness. Forty-nine patients (49.0%) were assigned to receive the intervention, and 51 (51.0%) were assigned to receive standard care. Hemoglobin concentration at 1 month after discharge was greater in patients receiving the intervention vs standard care (median [IQR], 12.2 [11.8-13.0] g/dL vs 11.5 [10.2-12.6] g/dL; adjusted mean difference, 0.69 [95% CI, 0.13-1.20] g/dL; P = .02).
Conclusions and relevance: This randomized clinical trial found that a multifaceted anemia prevention and treatment bundle was feasible, was well tolerated, and improved posthospitalization hemoglobin concentrations up to 3 months in critically ill adults. These findings can inform the design of future trials.
Trial registration: ClinicalTrials.gov Identifier: NCT05167734.
Conflict of interest statement
Figures


Comment in
- doi: 10.1001/jamanetworkopen.2025.2368
Similar articles
-
Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit.Cochrane Database Syst Rev. 2018 Mar 27;3(3):CD010754. doi: 10.1002/14651858.CD010754.pub2. Cochrane Database Syst Rev. 2018. PMID: 29582429 Free PMC article.
-
Exercise rehabilitation following intensive care unit discharge for recovery from critical illness.Cochrane Database Syst Rev. 2015 Jun 22;2015(6):CD008632. doi: 10.1002/14651858.CD008632.pub2. Cochrane Database Syst Rev. 2015. PMID: 26098746 Free PMC article.
-
Transfusion thresholds for guiding red blood cell transfusion.Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD002042. doi: 10.1002/14651858.CD002042.pub5. Cochrane Database Syst Rev. 2021. PMID: 34932836 Free PMC article.
-
Mobile App-Facilitated Collaborative Palliative Care Intervention for Critically Ill Older Adults: A Randomized Clinical Trial.JAMA Intern Med. 2025 Feb 1;185(2):173-183. doi: 10.1001/jamainternmed.2024.6838. JAMA Intern Med. 2025. PMID: 39680398 Clinical Trial.
-
Intensive case management for severe mental illness.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD007906. doi: 10.1002/14651858.CD007906.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2017 Jan 06;1:CD007906. doi: 10.1002/14651858.CD007906.pub3. PMID: 20927766 Free PMC article. Updated.
References
-
- Hébert PC, Wells G, Blajchman MA, et al. ; Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group . A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

