An Overview of Isavuconazole Clinical Use: A Multicentre Analysis of Indications, Exposure and Hepatic Safety
- PMID: 40153171
- PMCID: PMC12058921
- DOI: 10.1007/s40261-025-01432-z
An Overview of Isavuconazole Clinical Use: A Multicentre Analysis of Indications, Exposure and Hepatic Safety
Abstract
Background: Isavuconazole is a recent broad-spectrum triazole indicated for the treatment of invasive aspergillosis and mucormycosis when amphotericin B is inappropriate. However, limited information exists on its clinical use.
Objective: We set up a retrospective multicentre study to describe the clinical practice of isavuconazole including indications, exposure, and hepatic safety.
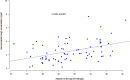
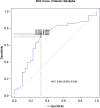
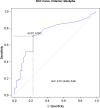
Methods: From January 2021 to June 2023, all patients who received isavuconazole and had at least one therapeutic drug monitoring (TDM) measurement, were included. To identify independent predictors of isavuconazole trough concentrations (Cmin), linear regression analyses were performed. Causal relationship between the occurrence of liver injury and isavuconazole was also analysed.
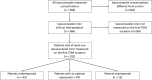
Results: Most of the included patients (n = 102) were admitted into haematology units (41.1% [n = 42]) or intensive care units (ICU) (30.4% [n = 31]). Aspergillosis (47.0% [n = 48]), mucormycosis (25.6% [n = 26]), and off-label empirical treatments (18.6% [n = 19]), were the three most common indications. About half of the patients (46.1% [n = 47]) had an optimal exposure, while 42.2% (n = 43) were underexposed, and 11.7% (n = 12) were overexposed. Albumin level on the day of TDM was a significant factor associated with an increase in isavuconazole Cmin (p = 0.010). Among the 11 patients who had liver test abnormalities, isavuconazole was discontinued in six (n = 6) patients and liver injury was attributable to isavuconazole in two (n = 2) patients.
Conclusions: This multicentre analysis highlighted the common use of isavuconazole as an off-label indication, as well as the frequent underexposure of patients to isavuconazole. Albumin on the day of TDM appeared to be an important factor driving isavuconazole exposure, especially in ICU patients.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Hospices Civils de Lyon. Conflicts of interest: Sylvain Goutelle is an Editorial Board member of Clinical Drug Investigation. Sylvain Goutelle was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions Ethics Approval: This was a non-interventional study with no additional procedure. Data were collected during routine patient care. This study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. This study was approved as an evaluation of professional practices by the Quality department of Lyon university hospital. Consent to Participate: Due to the retrospective nature of the study, formal consent was not required. Electronic records were under the auspice of the French National Committee for Data Protection and Freedom of Information (N°23–138). Consent for Publication: Not applicable. Availability of Data and Material: The data that support the findings of this study are not openly available due to protect study participant privacy. Code Availability: Not applicable. Authors’ Contributions: ALB, SG, and LF, designed the work. AD, AH, ALB, CC, CM, CMC, HLW, NM, PP, PR, and ACL, contributed to data acquisition. ALB, AM, FP, PP, SG, and TV, contributed to data analysis. ALB and AD wrote the original draft. All authors reviewed the manuscript critically and approved the final version. All authors agreed to be accountable for all aspects of the work.
Figures
References
-
- Brown GD, Ballou ER, Bates S, Bignell EM, Borman AM, Brand AC, et al. The pathobiology of human fungal infections. Nat Rev Microbiol. 2024;22:687–704. - PubMed
-
- Wu Y-J. Chapter 1 - Heterocycles and medicine: a survey of the heterocyclic drugs approved by the U.S. FDA from 2000 to present. In: Gribble GW, Joule JA, editors. Progress in heterocyclic chemistry [Internet]. Elsevier; 2012 [cited 2024 Jul 18]. p 1–53. Available from: https://www.sciencedirect.com/science/article/pii/B9780080968070000014. Accessed March 22, 2025.
-
- Cresemba | European Medicines Agency [Internet]. [cited 2023 Dec 14]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cresemba. Accessed March 22, 2025.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

