Dopaminergic neurons in the paraventricular hypothalamus extend the food consumption phase
- PMID: 40153459
- PMCID: PMC12002271
- DOI: 10.1073/pnas.2411069122
Dopaminergic neurons in the paraventricular hypothalamus extend the food consumption phase
Abstract
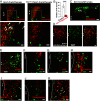
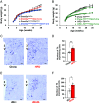
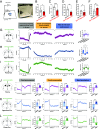
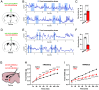
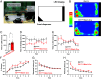
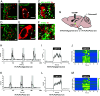
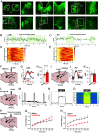
Feeding behavior is controlled by various neural networks in the brain that are involved in different feeding phases: Food procurement, consumption, and termination. However, the specific neural circuits controlling the food consumption phase remain poorly understood. Here, we investigated the roles of dopaminergic neurons in the paraventricular nucleus of the hypothalamus (PVH) in the feeding behavior in mice. Our results indicated that the PVH dopaminergic neurons were critical for extending the food consumption phase and involved in the development of obesity through epigenetic mechanisms. These neurons synchronized with proopiomelanocortin neurons during consumption, were stimulated by proopiomelanocortin activation, and projected to the lateral habenula (LHb), where dopamine receptor D2 was involved in the increase in food consumption. In addition, upregulated tyrosine hydroxylase (TH) expression in PVH was associated with obesity and indispensable for obesity induction in mice lacking Dnmt3a. Taken together, our results highlight the roles of PVH dopaminergic neurons in promoting food consumption and obesity induction.
Keywords: DNA methylation; dopamine; food intake; hypothalamus.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Comment in
-
Refining the role of the paraventricular hypothalamus in feeding behavior.Proc Natl Acad Sci U S A. 2025 May 13;122(19):e2506038122. doi: 10.1073/pnas.2506038122. Epub 2025 May 5. Proc Natl Acad Sci U S A. 2025. PMID: 40324098 Free PMC article. No abstract available.
References
-
- Barsh G. S., Schwartz M. W., Genetic approaches to studying energy balance: Perception and integration. Nat. Rev. Genet 3, 589–600 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- 15K20903/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21H03349/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22KF0061/MEXT | Japan Society for the Promotion of Science (JSPS)
- NA/Lotte Foundation
- NA/Mishima Kaiun Memorial Foundation
- NA/Japan Foundation for Pediatric Research (JPS)
- NA/Takeda Science Foundation (TSF)
- NA/Naito Foundation
- NA/kobayashi foundation
- NA/The Japan Science Society
- JPMJCR1921/MEXT | JST | Core Research for Evolutional Science and Technology (CREST)
- JP22zf0127007/Japan Agency for Medical Research and Development (AMED)
- NA/Manpei Suzuki Diabetes Foundation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

