RNAi screening of uncharacterized genes identifies promising druggable targets in Schistosoma japonicum
- PMID: 40153463
- PMCID: PMC11977999
- DOI: 10.1371/journal.ppat.1013014
RNAi screening of uncharacterized genes identifies promising druggable targets in Schistosoma japonicum
Abstract
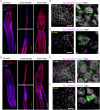
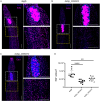
Schistosomiasis affects more than 250 million people worldwide and is one of the neglected tropical diseases. Currently, the treatment of schistosomiasis relies on a single drug-praziquantel-which has led to increasing pressure from drug resistance. Therefore, there is an urgent need to find new treatments. The development of genome sequencing has provided valuable information for understanding the biology of schistosomes. In the genome of Schistosoma japonicum, approximately 11% of the protein-coding sequences are uncharacterized genes (UGs) annotated as "hypothetical protein" or "protein of unknown function." These poorly understood genes have been unjustifiably neglected, although some may be essential for the survival of the parasites and serve as potential drug targets. In this study, we systematically mined the highly expressed UGs in both genders of this parasite throughout key developmental stages in their mammalian host, using our previously published S. japonicum genome and RNA-seq data. By employing in vitro RNA interference (RNAi), we screened 126 UGs that lack homologs in Homo sapiens and identified 8 that are essential for the parasite vitality. We further investigated two UGs, Sjc_0002003 and Sjc_0009272, which resulted in the most severe phenotypes. Fluorescence in situ hybridization demonstrated that both genes were expressed throughout the body without sex bias. Silencing either Sjc_0002003 or Sjc_0009272 reduced the cell proliferation in the body. Furthermore, in vivo RNAi indicated both genes are required for the growth and survival of the parasites in the mammalian host. For Sjc_0002003, we further characterize the underlying molecular cause of the observed phenotype. Through RNA-seq analysis and functional studies, we revealed that silencing Sjc_0002003 reduces the expression of a series of intestinal genes, including Sjc_0007312 (hypothetical protein), Sjc_0008276 (vha-17), Sjc_0002942 (PLA2G15), and Sjc_0003646 (SJCHGC09134 protein), leading to gut dilation. Our work highlights the importance of UGs in schistosomes as promising targets for drug development in the treatment of the schistosomiasis.
Copyright: © 2025 Xie et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

