Neuraminidase-specific antibodies drive differential cross-protection between contemporary FLUBV lineages
- PMID: 40153499
- PMCID: PMC11952091
- DOI: 10.1126/sciadv.adu3344
Neuraminidase-specific antibodies drive differential cross-protection between contemporary FLUBV lineages
Abstract
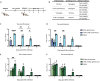
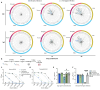
The two influenza B virus (FLUBV) lineages have continuously diverged from each other since the 1980s, with recent (post-2015) viruses exhibiting accelerated evolutionary rates. Emerging data from human studies and epidemiological models suggest that increased divergence in contemporary viruses may drive differential cross-protection, where infection with Yamagata lineage viruses provides limited immunity against Victoria lineage viruses. Here, we developed animal models to investigate the mechanisms behind asymmetric cross-protection between contemporary FLUBV lineages. Our results show that contemporary Victoria immunity provides robust cross-protection against the Yamagata lineage, whereas Yamagata immunity offers limited protection against the Victoria lineage. This differential cross-protection is driven by Victoria-elicited neuraminidase (NA)-specific antibodies, which show cross-lineage reactivity, unlike those from Yamagata infections. These findings identify a phenomenon in contemporary FLUBV that may help explain the recent disappearance of the Yamagata lineage from circulation, highlighting the crucial role of targeting NA in vaccination strategies to enhance cross-lineage FLUBV protection.
Figures

References
-
- Francis T. Jr., A new type of virus from epidemic influenza. Science 92, 405–408 (1940). - PubMed
-
- McCullers J. A., Hayden F. G., Fatal influenza B infections: Time to reexamine influenza research priorities. J. Infect. Dis. 205, 870–872 (2012). - PubMed
-
- Rosu M. E., Lexmond P., Bestebroer T. M., Hauser B. M., Smith D. J., Herfst S., Fouchier R. A. M., Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages. Proc. Natl. Acad. Sci. U.S.A. 119, e2211616119 (2022). - PMC - PubMed
-
- Rota P. A., Wallis T. R., Harmon M. W., Rota J. S., Kendal A. P., Nerome K., Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175, 59–68 (1990). - PubMed
-
- Sandt C. E., Bodewes R., Rimmelzwaan G. F., Vries R. D., Influenza B viruses: Not to be discounted. Future Microbiol. 10, 1447–1465 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

