Loss of GalNAc-T14 links O-glycosylation defects to alterations in B cell homing in IgA nephropathy
- PMID: 40153534
- PMCID: PMC12077892
- DOI: 10.1172/JCI181164
Loss of GalNAc-T14 links O-glycosylation defects to alterations in B cell homing in IgA nephropathy
Abstract
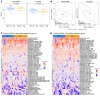
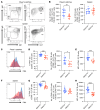
Aberrant O-glycosylation of the IgA1 hinge region is a characteristic finding in patients with IgA nephropathy (IgAN) and is thought to contribute to immune-complex formation and kidney injury. Other studies have suggested that abnormalities in mucosal immunity and lymphocyte homing are major contributors to disease. We identified a family with IgAN segregating a heterozygous predicted loss-of-function (LOF) variant in GALNT14, the gene encoding N-acetylgalactosaminyltransferase 14, one of the enzymes involved in mucin-type protein O-glycosylation. While GALNT14 is expressed in IgA1-producing cells, carriers of the LOF variant did not have altered levels of poorly glycosylated IgA1, suggesting other disease mechanisms. Investigation of Galnt14-null mice revealed elevated serum IgA levels and ex vivo IgA production by B cells. These mice developed glomerular IgA deposition with aging and after induction of sterile colitis. Galnt14-null mice also displayed an attenuated mucin layer in the colon and redistribution of IgA-producing cells from mucosal to systemic sites. Adoptive-transfer experiments indicated impaired homing of spleen-derived Galnt14-deficient B lymphocytes, resulting in increased retention in peripheral blood. These findings suggest that abnormalities in O-glycosylation alter mucosal immunity and B lymphocyte homing, pointing to an expanded role of aberrant O-glycosylation in the pathogenesis of IgAN.
Keywords: Genetic diseases; Genetics; Glycobiology; Immunoglobulins; Nephrology.
Figures





Comment in
- GALNT14 deficiency: connecting multiple links in the IgA nephropathy pathogenetic chain doi: 10.1172/JCI192687
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

