Intermittent versus Daily Therapy for Noncavitary Mycobacterium avium Complex Pulmonary Disease: An Open-Label Randomized Trial
- PMID: 40153596
- PMCID: PMC12329335
- DOI: 10.1513/AnnalsATS.202406-626OC
Intermittent versus Daily Therapy for Noncavitary Mycobacterium avium Complex Pulmonary Disease: An Open-Label Randomized Trial
Abstract
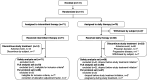
Rationale: Patients with noncavitary nodular bronchiectatic (NB) Mycobacterium avium complex pulmonary disease (MAC-PD) are treated intermittently three times per week, although no randomized controlled trials have been conducted comparing three times weekly with daily therapy. Objectives: To assess the tolerability, safety, and efficacy of intermittent versus daily treatment in patients with previously untreated noncavitary NB MAC-PD. Methods: In an open-label study, patients were randomly assigned to the intermittent therapy group receiving clarithromycin 1,000 mg, rifampicin 600 mg, and ethambutol 25 mg/kg (maximum 1,000 mg) three days per week or the daily therapy group receiving clarithromycin 800 mg, rifampicin 450 mg, and ethambutol 15 mg/kg (maximum 750 mg) daily for 1 year. The primary endpoint was the proportion of patients requiring modification of the initial treatment regimen. Results: Twenty-one Japanese hospitals participated in the study, enrolling 141 patients between May 2019 and December 2021. The full analysis set included 138 participants (intermittent therapy = 70; daily therapy = 68). There were no significant differences between the intermittent and daily therapy groups in terms of the regimen modification rate (20.0% [14 of 70] vs. 33.8% [23 of 68]; adjusted odds ratio, 0.48; 95% confidence interval, 0.22 to 1.05; P = 0.06) or culture conversion (70.3% vs. 80.0%; P = 0.53), time to culture conversion (28.0 vs. 28.5 d; P = 0.89), improvement in chest CT findings (60.9% vs. 71.0%; P = 0.30), or clarithromycin resistance development (1.4% vs. 0%; P = 1.00). Elevated aspartate aminotransferase (16.9% vs. 41.2%; P = 0.003) and alanine aminotransferase (18.3% vs. 44.1%; P = 0.002) were more common in the daily treatment group, whereas elevated bilirubin (11.3% vs. 1.5%; P = 0.04) and dysgeusia (14.1% vs. 1.5%; P = 0.01) were more common in the intermittent treatment group. The daily treatment group exhibited a greater absolute change in the 36-Item Short Form Health Survey physical aspect score (-2.5 points) than the intermittent treatment group (2.1 points) (P = 0.01). Conclusions: Intermittent treatment was not significantly better tolerated than daily treatment for noncavitary NB MAC-PD. However, further studies with larger numbers of patients are needed. Clinical trial registered with https://jrct.mhlw.go.jp/en-top (jRCTs031190008).
Keywords: adverse drug reaction; health-related quality of life; intermittent treatment; nontuberculous mycobacteria.
Figures

References
-
- Dahl VN, Mølhave M, Fløe A, van Ingen J, Schön T, Lillebaek T, et al. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis . 2022;125:120–131. - PubMed
-
- Morimoto K, Hasegawa N, Izumi K, Namkoong H, Uchimura K, Yoshiyama T, et al. A laboratory-based analysis of nontuberculous mycobacterial lung disease in Japan from 2012 to 2013. Ann Am Thorac Soc . 2017;14:49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

