A phase 2a trial of the IL-33 monoclonal antibody tozorakimab in patients with COPD: FRONTIER-4
- PMID: 40154559
- PMCID: PMC12256803
- DOI: 10.1183/13993003.02231-2024
A phase 2a trial of the IL-33 monoclonal antibody tozorakimab in patients with COPD: FRONTIER-4
Abstract
Background: Interleukin-33 may have a role in COPD pathobiology. FRONTIER-4 (NCT04631016) investigated tozorakimab (an anti-interleukin-33 monoclonal antibody) in patients with moderate-to-severe COPD with chronic bronchitis receiving dual or triple inhaled therapy.
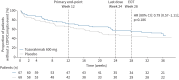
Methods: FRONTIER-4 was a phase 2a, randomised, double-blind, placebo-controlled study. Patients received tozorakimab 600 mg or placebo subcutaneously every 4 weeks for 24 weeks. The primary end‑point was change in pre-bronchodilator forced expiratory volume in 1 s (FEV1) from baseline to week 12. Secondary outcomes included post-bronchodilator FEV1, time-to-first COPD composite exacerbation event and safety.
Results: The intent-to-treat population included 135 patients (tozorakimab, n=67; placebo, n=68). At week 12 in the intent-to-treat population, tozorakimab showed a greater increase, although nonsignificant, from baseline in pre-bronchodilator FEV1 (least-squares mean difference (LSMD) 24 mL, 80% confidence interval (CI) -15-63 mL, p=0.216) and a significantly greater increase in post-bronchodilator FEV1 (LSMD 67 mL, 80% CI 17-116 mL, p=0.044) when compared with placebo. At week 12 in a prespecified subgroup of patients with at least two prior exacerbations, tozorakimab also showed improvements versus placebo in change from baseline in pre-bronchodilator FEV1 (LSMD 69 mL, 80% CI 9-130 mL, p=0.072) and post-bronchodilator FEV1 (LSMD 124 mL, 80% CI 47-201 mL, p=0.020). Tozorakimab did not significantly reduce the risk of COPD composite exacerbation events (hazard ratio 0.79, 80% CI 0.57-1.11, p=0.186) in the intent-to-treat population, although there were greater effects in patients with at least two prior exacerbations (hazard ratio 0.61, 80% CI 0.37-1.00). Results were similar in former and current smokers. Tozorakimab was well tolerated.
Conclusion: Although the primary end-point was not met in the intent-to-treat population, tozorakimab showed positive efficacy signals versus placebo in a subgroup of patients with COPD with a high risk of exacerbations.
Copyright ©The authors 2025.
Conflict of interest statement
Conflicts of interest: D. Singh has received personal fees from Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CONNECT Biopharma, Covis Pharma, CSL Behring, DevPro Biopharma, Elpen, Empirico, EpiEndo Pharmaceuticals, Genentech, Generate Biomedicines, GSK, Glenmark, Kamada, Kinaset Therapeutics, Kymera Therapeutics, Menarini, MicroA, OM Pharma, Orion Pharma, Pieris Pharmaceuticals, Pulmatrix, Revolo Biotherapeutics, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva Pharmaceuticals, Theravance Biopharma, Upstream Bio and Verona Pharma. P. Guller, F. Reid, S. Doffman, U. Seppälä, I. Psallidas, R. Moate, J. Kiraga, E. Jimenez, D. Brooks, A. Kelly, L.H. Nordenmark, M.W. Sadiq, C. Kell, M.G. Belvisi and H. Pandya are employees of AstraZeneca and may hold stock or stock options in AstraZeneca. R. Smith was an employee of AstraZeneca at the time the study was conducted and may hold stock or stock options in AstraZeneca. L.M. Caballero has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Sanofi.
Figures




Comment in
-
IL-33, inflammation and mucus in COPD: the final FRONTIER?Eur Respir J. 2025 Jul 14;66(1):2500831. doi: 10.1183/13993003.00831-2025. Print 2025 Jul. Eur Respir J. 2025. PMID: 40659469 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
