Pirfenidone in post-COVID-19 pulmonary fibrosis (FIBRO-COVID): a phase 2 randomised clinical trial
- PMID: 40154560
- PMCID: PMC12018760
- DOI: 10.1183/13993003.02249-2024
Pirfenidone in post-COVID-19 pulmonary fibrosis (FIBRO-COVID): a phase 2 randomised clinical trial
Abstract
Background: Patients with severe COVID-19 may develop lung fibrosis. Pirfenidone is an anti-fibrotic drug approved for idiopathic pulmonary fibrosis. The efficacy and safety of pirfenidone in patients with fibrotic interstitial lung changes after recovery from severe COVID-19 pneumonia were evaluated.
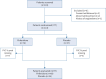
Methods: This was a phase 2, double-blind, placebo-controlled, Spanish multicentre clinical trial. Patients were randomised to receive pirfenidone or placebo (2:1) for 24 weeks. The primary end-point was the proportion of patients that improved, considered when percentage change in forced vital capacity (FVC) was ≥10% and/or any reduction in the fibrotic score on chest high-resolution computed tomography (HRCT). Secondary end-points included health-related quality of life (HRQoL), exercise capacity and drug safety profile.
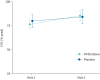
Results: From 119 eligible patients, 113 were randomised and 103 were analysed (pirfenidone n=69 and placebo n=34). Most patients were male (73.5%) and were receiving low-dose prednisone; mean age was 63.7 years and mean body mass index was 29 kg·m-2. The percentage of patients that improved was similar in the pirfenidone and placebo groups (79.7% versus 82.3%, respectively). The mean predicted FVC increased by 12.74±20.6% with pirfenidone and 4.35±22.3% with placebo (p=0.071), and the HRCT (%) fibrotic score decreased by 5.44±3.69% with pirfenidone and 2.57±2.59% with placebo (p=0.52). Clinically meaningful improvement in HRQoL was not statistically different (55.2% in the pirfenidone group and 39.4% in the placebo group). Exercise capacity, adverse events and hospitalisations were similar between groups. No deaths were reported.
Conclusions: The overall improvements in lung function and HRCT fibrotic score after 6 months with pirfenidone were not significantly different than with placebo.
Copyright ©The authors 2025.
Conflict of interest statement
Conflicts of interest: M. Molina-Molina has received grants and fees for scientific advice from Boehringer Ingelheim, Roche, Ferrer and Veracyte, outside the submitted work. D. Castillo reports personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Boehringer Ingelheim, grants from Fujirebio, and personal fees from Veracyte, outside the submitted work. J. Rigual Bobillo has received grants and fees for scientific advice from Boehringer Ingelheim, Roche, Daiichi Sankyo and AstraZeneca, outside the submitted work. The other authors have no conflicts of interest to declare.
Figures

Comment in
-
If it's broken, fix it: clinical trials need a paradigm shift.Eur Respir J. 2025 Apr 24;65(4):2500331. doi: 10.1183/13993003.00331-2025. Print 2025 Apr. Eur Respir J. 2025. PMID: 40274296 No abstract available.
-
Pirfenidone in post-COVID-19 pulmonary fibrosis.Eur Respir J. 2025 Sep 17;66(3):2501271. doi: 10.1183/13993003.01271-2025. Print 2025 Sep. Eur Respir J. 2025. PMID: 40962426 Free PMC article.
