Quality of life, neurocognitive functioning, psychological issues, sexuality and comorbidity more than 2 years after commencing immune checkpoint inhibitor treatment
- PMID: 40154959
- PMCID: PMC11956391
- DOI: 10.1136/jitc-2024-011168
Quality of life, neurocognitive functioning, psychological issues, sexuality and comorbidity more than 2 years after commencing immune checkpoint inhibitor treatment
Abstract
Background: Increasing numbers of patients diagnosed with advanced cancer survive long-term after treatment with immune checkpoint inhibitors (ICIs). To design adequate interventions for these survivors, knowledge regarding quality of life (QOL) and its association with long-term and late effects of ICI treatment is required. Therefore, this study aimed to evaluate QOL, neurocognitive function, psychological issues, sexuality, and comorbidities in patients surviving at least 2 years after commencing ICI treatment.
Methods: We performed a cross-sectional study in patients with stage III-IV melanoma, non-small cell lung cancer (NSCLC), urothelial cell carcinoma (UCC), or renal cell carcinoma (RCC) who survived at least 2 years after the start of ICIs. We assessed QOL, neurocognitive function, psychological issues, sexual function and comorbidity in survivors. Additionally, we evaluated QOL of informal caregivers.
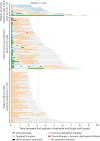
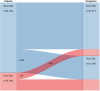
Results: 132 survivors (70 melanoma, 50 NSCLC, 12 UCC or RCC) and 80 caregivers were included. Median age was 65 years (range 30-85) and 50 survivors were women (38%). Median time since start and cessation of ICI treatment was 33 (range 21-91) and 18 (range 0-68) months, respectively. Average survivor QOL was comparable to the reference population, but 37 (28%) survivors had poor QOL. Depression and anxiety were negatively correlated with all QOL domains. Although immune-related adverse events were common, there was no association with lower QOL. Caregiver and survivor QOL were only weakly related. Neurocognitive concerns and formally tested neurocognitive impairment were present in 22 (17%) and 13 (15%) survivors, respectively, and were not associated with a diagnosis of brain metastases. Men had a high prevalence of erectile dysfunction and low sexual satisfaction. Half of the survivors met the criteria for the metabolic syndrome.
Conclusions: At least 2 years after the start of ICI treatment, one-quarter of cancer survivors had a clinically relevant lower QOL. This was associated with symptoms of depression and anxiety, but not with immune-related adverse events. Sexual issues and metabolic syndrome are prevalent. Survivorship care should address these issues in this population.
Keywords: COGNITION; Immune Checkpoint Inhibitors; Immune related adverse event - irAE; QUALITY OF LIFE; Survivorship.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MJ reports fees for advisory board Pierre Fabre, Regeneron and Merck, paid to institution. TJNH reports research grants from Roche, AstraZeneca, and BMS, paid to the institution. Other authors declare no relevant conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
