Long-term follow-up of BCMA CAR-T cell therapy in patients with relapsed/refractory multiple myeloma
- PMID: 40154960
- PMCID: PMC11956354
- DOI: 10.1136/jitc-2024-010687
Long-term follow-up of BCMA CAR-T cell therapy in patients with relapsed/refractory multiple myeloma
Abstract
Background: B-cell maturation antigen (BCMA)-targeting chimeric antigen receptor (CAR) T-cell immunotherapy has shown promising results in the treatment of relapsed or refractory multiple myeloma (R/RMM). This study presents the updated long-term outcomes from our center.
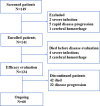
Methods: Between July 30, 2018, and September 27, 2023, 141 patients with R/RMM who received BCMA CAR-T therapy were enrolled. Patients underwent conditioning chemotherapy with cyclophosphamide and fludarabine, followed by BCMA CAR-T cell infusion at a median dose of 2.36×106 cells/kg. The study evaluated overall response rates, long-term efficacy, safety profiles, and their associations with clinical and disease characteristics.
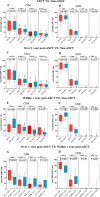
Results: At a median follow-up of 20.2 months, the safety profile of the therapy was manageable. Grade 3/4 cytokine release syndrome occurred in 36.2% of patients, with no cases of severe neurotoxicity reported. 1-month post-infusion, grade ≥3 anemia persisted in 39.6% of patients, while neutropenia (43.3%) and thrombocytopenia (52.2%) were observed. The objective response rate (ORR) among evaluable patients was 94.8%, with 50.7% achieving a complete response (CR). The 4-year progression-free survival and overall survival rates were 37.4% (95% CI, 29.1% to 48.1%) and 63.2% (95% CI, 54.8% to 72.8%), respectively, with survival curves showing gradual flattening over time. Patients with a history of autologous stem cell transplantation (ASCT) and those with extramedullary disease demonstrated significantly inferior efficacy and survival outcomes. Peak CAR-T cell expansion was positively correlated with ORR (p<0.001) and CR (p<0.001). Notably, patients with prior ASCT exhibited significantly lower CAR-T cell expansion compared with those without prior ASCT (p<0.001). Immunophenotypic analysis of infused CAR-T cells demonstrated impaired fitness in patients who received ASCT in the past year.
Conclusions: BCMA CAR-T therapy in patients with R/RMM results in significant and sustained responses, with a manageable safety profile on a large scale. Prior ASCT and extramedullary disease represent adverse prognostic factors. Patients with a history of ASCT demonstrate limited peak CAR-T cell expansion.
Keywords: Immunotherapy; Multiple Myeloma.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: Authors YZ and AHC are employed by Shanghai YaKe Biotechnology. The remaining authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
