Germline prediction of immune checkpoint inhibitor discontinuation for immune-related adverse events
- PMID: 40154961
- PMCID: PMC11956315
- DOI: 10.1136/jitc-2024-011273
Germline prediction of immune checkpoint inhibitor discontinuation for immune-related adverse events
Abstract
Introduction: Immune checkpoint inhibitors (ICIs) can yield remarkable clinical responses in subsets of patients with solid tumors, but they also commonly cause immune-related adverse events (irAEs). The predictive features of clinically severe irAEs leading to cessation of ICIs have yet to be established. Given the similarities between irAEs and autoimmune diseases, we sought to investigate the association of a germline polygenic risk score for autoimmune disease and discontinuation of ICIs due to irAEs.
Methods: The Genetics of immune-related adverse events and Response to Immunotherapy (GeRI) cohort comprises 1302 patients with non-small cell lung cancer (NSCLC) who received ICI therapy between 2009 and 2022 at four academic medical centers. We used a published polygenic risk score for autoimmune diseases (PRSAD) in the general population and validated it in the All of Us. We then assessed the association between PRSAD and cessation of ICI therapy due to irAEs in the GeRI cohort, using cause-specific and Fine-Gray subdistribution hazard models. To further understand the differential effects of type of therapy on the association between PRSAD and cessation of ICI due to irAEs, we conducted a stratified analysis by type of ICI therapy.
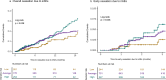
Results: Using a competing risk model, we found an association between PRSAD and ICI cessation due to irAEs (HR per SD=1.24, p=0.004). This association was particularly strong in patients who had ICI cessation due to irAEs within 3 months of therapy initiation (HR per SD=1.40, p=0.005). Individuals in the top quintile of PRSAD had 4.8% ICI discontinuation for irAEs by 3 months, compared with 2% discontinuation by 3 months among patients in the bottom quintile (log-rank p=0.03). In addition, among patients who received combination programmed cell death protein-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors and cytotoxic T-lymphocyte associated protein 4 (CTLA4) inhibitors, ICI discontinuation for irAEs by 3 months occurred in 4 of the 13 patients (30.8%) with high PRSAD genetic risk (top quintile) versus 3 of 21 patients (14.3%) with low PRSAD genetic risk (bottom quintile).
Conclusions: We demonstrate an association between a polygenic risk score for autoimmune disease and early ICI discontinuation for irAEs. Our results suggest that germline genetics may be used as an adjunctive tool for risk stratification around ICI clinical decision-making in solid tumor oncology.
Keywords: Genetic; Immune Checkpoint Inhibitor; Immune related adverse event - irAE; Lung Cancer.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

Update of
-
Germline prediction of immune checkpoint inhibitor discontinuation for immune-related adverse events.medRxiv [Preprint]. 2024 Jun 11:2024.06.10.24308518. doi: 10.1101/2024.06.10.24308518. medRxiv. 2024. Update in: J Immunother Cancer. 2025 Mar 28;13(3):e011273. doi: 10.1136/jitc-2024-011273. PMID: 38947092 Free PMC article. Updated. Preprint.
References
-
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–86. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- S10 OD017985/OD/NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 CA227466/CA/NCI NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- OT2 OD025315/OD/NIH HHS/United States
- OT2 OD026552/OD/NIH HHS/United States
- OT2 OD026549/OD/NIH HHS/United States
- OT2 OD025337/OD/NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA233259/CA/NCI NIH HHS/United States
- OT2 OD025277/OD/NIH HHS/United States
- OT2 OD026555/OD/NIH HHS/United States
- RC2 GM092618/GM/NIGMS NIH HHS/United States
- U01 HG006378/HG/NHGRI NIH HHS/United States
- OT2 OD025276/OD/NIH HHS/United States
- OT2 OD026554/OD/NIH HHS/United States
- U24 OD023163/OD/NIH HHS/United States
- U01 CA253560/CA/NCI NIH HHS/United States
- OT2 OD026556/OD/NIH HHS/United States
- U01 FD005978/FD/FDA HHS/United States
- U24 OD023176/OD/NIH HHS/United States
- OT2 OD026548/OD/NIH HHS/United States
- U2C OD023196/OD/NIH HHS/United States
- T32 CA009207/CA/NCI NIH HHS/United States
- S10 RR025141/RR/NCRR NIH HHS/United States
- OT2 OD026551/OD/NIH HHS/United States
- U24 OD023121/OD/NIH HHS/United States
- R01 CA251758/CA/NCI NIH HHS/United States
- OT2 OD026550/OD/NIH HHS/United States
- R01 HD074711/HD/NICHD NIH HHS/United States
- OT2 OD026553/OD/NIH HHS/United States
- K12 DK133995/DK/NIDDK NIH HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- OT2 OD023205/OD/NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- R01 NS032830/NS/NINDS NIH HHS/United States
- U01 HG004798/HG/NHGRI NIH HHS/United States
- OT2 OD026557/OD/NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- P01 CA129243/CA/NCI NIH HHS/United States
- OT2 OD023206/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
