Mavacamten maintenance dose determination: insights into individualised therapy for hypertrophic cardiomyopathy
- PMID: 40154977
- PMCID: PMC11956304
- DOI: 10.1136/openhrt-2025-003192
Mavacamten maintenance dose determination: insights into individualised therapy for hypertrophic cardiomyopathy
Abstract
Aims: Mavacamten, the first approved myosin inhibitor for symptomatic obstructive hypertrophic cardiomyopathy (oHCM), addresses hypercontractility and left ventricular outflow tract (LVOT) obstruction. This study evaluates real-world experience with mavacamten, focusing on maintenance dose determination to optimise individual therapy and enhance patient safety.
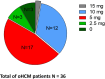
Methods: 36 patients with symptomatic oHCM who completed the initiating phase of mavacamten therapy were analysed. CYP2C19 genetic testing determined metabolic status prior to treatment. Echocardiographic measurements (eg, LVOT gradient, left atrial volume index, left ventricular ejection fraction (LVEF) and E/E') and biomarkers (high-sensitivity troponin I, N-terminal pro B-type natriuretic peptide (NT-proBNP)) were assessed at baseline and after 3 months. Clinical status was evaluated using New York Heart Association (NYHA) class and Kansas City Cardiomyopathy Questionnaire (KCCQ) score.
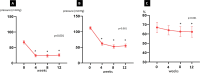
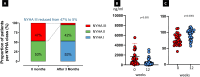
Results: The mean age of patients was 60.6±12.1, and all had normal CYP2C19 metabolic status. LVEF was 68% (IQR 8) at baseline and decreased mildly to 60.5% (IQR 7.25; p=0.0004) without cases dropping below 50%. Resting and provoked LVOT gradients decreased from 65 mm Hg (IQR 43.75) and 105 mm Hg (IQR 36.25) to 12 mm Hg (IQR 15.5; p<0.001) and 52.5 mm Hg (IQR 46.5; p<0.001), respectively. NT-proBNP and high-sensitivity troponin I decreased significantly from 1040 ng/mL (IQR 1255) to 285 ng/mL (IQR 483; p=0.0005) and from 11 ng/mL (IQR 15.5) to 10 ng/mL (IQR 5; p<0.0001). Diastolic function improved slightly; and clinically, patients improved significantly, with improvement in NYHA class and increase in KCCQ score. Mean time to reach maintenance dose was 14 weeks, with the necessity of dose adjustments in more than 50% of cases.
Conclusion: Mavacamten therapy is safe and effective in the initiating phase. Determination of starting and maximum dose is based on CYP2C19 metabolic status, while individualised dose adjustments are guided by echocardiographic response to optimise patient safety.
Keywords: Cardiomyopathies; Cardiomyopathy, Hypertrophic; Heart Failure.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SS received travel expenses and speaker honoraria from Bristol Myers Squibb. VS has received research funding from Bristol Myers Squibb unrelated to this work.
Figures
References
-
- Ommen SR, Ho CY, Asif IM, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1239–311. doi: 10.1161/CIR.0000000000001250. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
