Effectiveness of intrapartum azithromycin to prevent infections in planned vaginal births in low-income and middle-income countries: a post-hoc analysis of data from a multicentre, randomised, double-blind, placebo-controlled trial
- PMID: 40155106
- PMCID: PMC11950427
- DOI: 10.1016/S2214-109X(24)00562-X
Effectiveness of intrapartum azithromycin to prevent infections in planned vaginal births in low-income and middle-income countries: a post-hoc analysis of data from a multicentre, randomised, double-blind, placebo-controlled trial
Abstract
Background: In 2023, the Azithromycin Prevention in Labor Use (A-PLUS) trial showed intrapartum azithromycin reduces maternal sepsis or death in women with planned vaginal delivery in low-resource settings, but whether it reduces maternal infection is unknown. We aimed to evaluate the effectiveness of intrapartum azithromycin in reducing maternal infection.
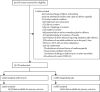
Methods: We performed a post-hoc analysis of the multicentre, facility-based, randomised, double-blind, placebo-controlled A-PLUS trial. This trial compared prophylactic intrapartum single oral dose of 2 g azithromycin versus placebo on maternal morbidity and mortality in low-resource settings in southeast Asia and Africa from Sept 9, 2020, to Aug 18, 2022. The trial enrolled women in labour at 28 weeks' gestation (or later) at eight sites in the Democratic Republic of the Congo, Kenya, Zambia, Bangladesh, India, Pakistan, and Guatemala and found that azithromycin reduced the incidence of maternal sepsis or death. The primary outcome of the present analysis was the incidence of any maternal infection in the azithromycin versus placebo groups, which was defined as one or more of these infections after randomisation: chorioamnionitis, endometritis, perineal or caesarean wound infection, abdominopelvic abscess, mastitis or breast abscess, and other infections. Any neonatal infection was also analysed. All analyses were by intention to treat in all those with data available for that outcome. Relative risks (RRs) and 95% CIs were estimated with a Poisson model adjusted for treatment group and site. Subgroup analyses included a two-way interaction test between intervention group and subgroup. A-PLUS was registered at ClinicalTrials.gov, number NCT03871491.
Findings: 29 278 women were randomly assigned to groups: 14 590 to receive azithromycin, 14 688 to receive placebo. Baseline characteristics were similar between the azithromycin and placebo groups (43·3% vs 43·4% primiparous, 8·5% vs 8·7% high risk for infection). The presence of any maternal infection occurred less often in the azithromycin group (580 [4·0%] of 14 558) compared with the placebo group (824 [5·6%] of 14 661 women; RR 0·71, 95% CI 0·64-0·79, p<0·0001). Any neonatal infection did not differ between treatment groups. Adverse events were not detected.
Interpretation: Among women planning vaginal delivery, this analysis provides evidence indicating that intrapartum azithromycin is associated with a lower incidence of maternal infections than placebo.
Funding: The Eunice Kennedy Shriver National Institute of Child Health and Human Development and Bill and Melinda Gates Foundation via Foundation of National Institutes of Health.
Translations: For the French and Spanish translations of the abstract see Supplementary Materials section.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests WAC has received support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the Bill & Melinda Gates Foundation via Foundation of National Institutes of Health (NIH) for this study and manuscript. WAC has received grants from the Thrasher Research Fund, the National Health, Lung, and Blood Institute, and the American Heart Association. WAC has also participated in Enhancing Quality and Access to Achieve Equitable Maternal and Infant Health, Enhanced glucose Monitoring to improve Pregnancy Outcomes for Women Requiring medications for gestational diabetes, National Institute of Arthritis and Musculoskeletal and Skin Diseases Salmon Study, and Increased Milk Protein to Accrue Critical Tissue. ATNT received support from the NIH, NICHD, and the Bill & Melinda Gates Foundation via Foundation of NIH for this study; additionally, he has received grants from Mirvie and American Heart Association. All authors received support for this manuscript from the NICHD and Foundation for the NIH.
Figures



Comment in
-
The promise of antibiotic-led reduction in maternal infection.Lancet Glob Health. 2025 Apr;13(4):e604-e605. doi: 10.1016/S2214-109X(25)00051-8. Lancet Glob Health. 2025. PMID: 40155093 No abstract available.
References
-
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. - PubMed
-
- WHO . World Health Organization; 2017. Statement on maternal sepsis.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

