Donanemab: Appropriate use recommendations
- PMID: 40155270
- PMCID: PMC12180672
- DOI: 10.1016/j.tjpad.2025.100150
Donanemab: Appropriate use recommendations
Abstract
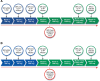
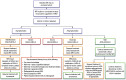
Donanemab (Kisunla®), an IgG1 monoclonal antibody targeting N-terminal pyroglutamate-modified forms of amyloid-β, is approved in the United States for treatment of early symptomatic Alzheimer's disease (AD). Appropriate Use Recommendations (AUR) were developed to guide the implementation of donanemab in real-world practice, prioritizing safety considerations and opportunity for effectiveness. The AUR were developed by the AD and Related Disorders Therapeutic Workgroup by consensus, integrating available data and expert opinion. Appropriate candidates for donanemab treatment include persons with mild cognitive impairment or mild dementia due to AD (Clinical Stages 3-4, MMSE 20-30) who have biomarker confirmation of AD pathology by PET or CSF. Tau PET is not required for eligibility. Apolipoprotein E (APOE) genotyping should be performed prior to treatment to inform an individual's risk of developing Amyloid-Related Imaging Abnormalities (ARIA). Pre-treatment MRI should be obtained no more than 12 months prior to treatment. Patients with findings of >4 cerebral microbleeds, cortical superficial siderosis or a major vascular contribution to cognitive impairment should be excluded from treatment. The decision to initiate therapy should be grounded in a shared decision-making process that emphasizes the patient's values and goals of care. Donanemab is administered as a monthly intravenous infusion. Surveillance MRIs to evaluate for ARIA should be performed prior to the 2nd, 3rd, 4th and 7th infusions, prior to the 12th dose in higher risk individuals, and at any time ARIA is suspected clinically. Clinicians may consider discontinuing treatment if amyloid clearance is demonstrated by amyloid PET, typically obtained 12-18 months after initiating treatment.
Keywords: Amyloid-targeting therapies; Antiamyloid monoclonal antibodies; Appropriate use recommendations; Donanemab; Expert guidelines.
Copyright © 2025 The Author(s). Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Rabinovici has received research support from Avid Radiopharmaceuticals, Eli Lilly, Genentech, GE Healthcare and Life Molecular Imaging. He has served as a paid scientific advisor for Alector, Avid Radiopharmaceuticals, Bristol Myers Squibb, C2N, Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk, Roche. He has received speaking honoraria from Peerview and Medscape. He is an Associate Editor for JAMA and JAMA Neurology. Dr. Selkoe is a director of Prothena Biosciences and an ad hoc consultant and speaker for Eisai and ad hoc consultant to Roche. Dr. Schindler has served on advisory boards and/or as a speaker for Eisai, Eli Lilly, Novo Nordisk. Dr. Aisen has research grants from Eli Lilly and Eisai, and consults with Merck, Roche, Genentech, Abbvie, Biogen, ImmunoBrain Checkpoint, AltPep, Alector, Arrowhead and Neurimmune. He is an Editor for JPAD. Dr. Apostolova has received grant support from Avid Radiopharmaceuticals, Life Molecular Imaging, Roche Diagnostics, Eli Lilly. She has consulted for Biogen, Two Labs, IQVIA, Genentech, Siemens, Corium, Eli Lilly, GE Healhcare, Eisai, Roche Diagnostics, Alnylam, Otsuka. Dr. Atri over the last 15 years, has received honoraria or support for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, AriBio, Axovant, AZ Therapies, Axsome, Biogen, Eisai, Forest, Grifols, JOMDD, Lantheus, Life Molecular, Lundbeck (in partnership with Otsuka), Merck, Novo Nordisk, ONO, Prothena, Qynapse, Roche, Genentech, Sunovion, Suven, Synexus, and Vaxxinity. He has served as a consultant to Biogen, Eisai, Prothena and Roche/Genentech and has served as a site-PI (institutional contract) for clinical trials sponsored by Biogen, Eisai, and Lilly. He served as project arm leader for DIAN-TU gantenerumab OLE study (WUSTL with Roche/Genentech). He currently serves as site PI for the USC/ATRI/ACTC & Eisai AHEAD 3–45 study, Project Arm Leader for the DIAN-TU ART study (lecanemab DIAD Amyloid Plaque Reduction and Prevention study), and Global Coordinating PI for the Advance Study (Life Molecular Imaging PI-2620 histopathology study). At his previous institution. He served as site PI for the Biogen EMERGE study. He receives royalties from Oxford University Press. Dr. Greenberg has consulted for Eli Lilly. Dr. Hendrix is owner of Pentara, and consults with dozens of companies in the Alzheimer's space, including Eli Lilly Dr. Petersen has served as a consultant for Roche, Genentech, Eli Lilly, Eisai, Novartis, Novo Norodisk. He receives royalties from Oxford University Press and UpToDate and has provided educational services for Medscape. Dr. Salloway has received grant support from Janssen, Eisai, Eli Lilly, Biogen and Cognition. He has provided consultation to Eli Lilly, Eisai, Biogen, Merck, BMS, Alector, Neurophet, Icometrix, Cognition Therapeutics, Biohaven, Novo Nordisk, Roche, Abbvie, Genentech, Acumen and Kisbee. He is an Associate Editor of the Journal of Prevention of Alzheimer's Disease and Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. Dr. Cummings has provided consultation to Acadia, Acumen, ALZpath, Annovis, Aprinoia, Artery, Biogen, Biohaven, BioXcel, Bristol-Myers Squib, Eisai, Fosun, GAP Foundation, Green Valley, Janssen, Karuna, Kinoxis, Lighthouse, Lilly, Lundbeck, LSP/eqt, Mangrove Therapeutics, Merck, MoCA Cognition, New Amsterdam, Novo Nordisk, onocC4, Optoceutics, Otsuka, Oxford Brain Diagnostics, Praxis, Prothena, ReMYND, Roche, Scottish Brain Sciences, Signant Health, Simcere, sinaptica, T-Neuro, TrueBinding, and Vaxxinity pharmaceutical, assessment, and investment companies. Dr. Cummings is supported by NIGMS grant P20GM109025; NIA R35AG71476; NIA R25AG083721–01; NINDS RO1NS139383; Alzheimer's Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; Joy Chambers-Grundy Endowment.
Figures
References
-
- Demattos R.B., et al. A plaque-specific antibody clears existing beta-amyloid plaques in Alzheimer's disease mice. Neuron. 2012;76(5):908–920. - PubMed
-
- Mintun M.A., et al. Donanemab in early Alzheimer's Disease. N Engl J Med. 2021;384(18):1691–1704. - PubMed
-
- FDA, U.S. 2023 [cited 2024 December 31]; Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment....
-
- Mori H., et al. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992;267(24):17082–17086. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

