S2303: phase II/III trial of paclitaxel + ramucirumab ± nivolumab in gastric and esophageal adenocarcinoma (PARAMUNE)
- PMID: 40155326
- PMCID: PMC12051544
- DOI: 10.1080/14796694.2025.2485020
S2303: phase II/III trial of paclitaxel + ramucirumab ± nivolumab in gastric and esophageal adenocarcinoma (PARAMUNE)
Abstract
Keywords: Immunotherapy; anti-programmed death-1; combined positive score; gastric, gastroesophageal junction, or esophageal cancer; paclitaxel; ramucirumab; vascular endothelial growth factor.
Plain language summary
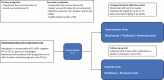
Cancer of the stomach and esophagus is aggressive with poor patient outcomes. Historically, the treatment for such patients has involved the use of combined chemotherapy. In recent years, the addition of immunotherapy to the treatment of patients with advanced cancers of the stomach and esophagus has led to patients living longer with a longer period without tumor growth. However, whether continuing the immunotherapy after the tumor has grown while on treatment is beneficial or not is a question that has not been answered. The standard treatment after patients’ tumor has grown on their initial treatment involves a drug called ramucirumab, which blocks the ability of cancer cells to create abnormal blood vessels around itself to support its own growth. Recent research suggests that drugs like ramucirumab make immunotherapy more effective. To test whether continuing immunotherapy in combination with this “second-line” treatment that includes ramucirumab, we have designed a study to compare whether one group who will receive immunotherapy will live longer versus another group who will not receive immunotherapy.
Conflict of interest statement
Anwaar Saeed reports consulting or advisory board role with AstraZeneca, Bristol-Myers Squibb, Merck, Exelixis, Pfizer, Xilio therapeutics, Taiho, Amgen, Autem therapeutics, KAHR medical, Arcus therapeutics, Regeneron, Replimune and Daiichi Sankyo; institutional research funding from AstraZeneca, Bristol-Myers Squibb, Merck, Clovis, Exelixis, Actuate therapeutics, Incyte Corporation, Daiichi Sankyo, Five prime therapeutics, Amgen, Innovent biologics, Dragonfly therapeutics, Oxford Biotherapeutics, Replimune, Phanes therapeutics, Arcus therapeutics, Regeneron and KAHR medical. Dan Duda reports receiving research grants from BMS, Exelixis, Surface Oncology, and Bayer. Riha Vaidya reports current employment at Flatiron Health. Flavio Rocha reports consultant for AZ and BetaGlue. Rachael A. Safyan reports institutional research funding from Replimune, Exelixis, and Verastem and consulting or advisory board from Mirati, Guardant, Agenus, Ipsen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2 - DOI - PMC - PubMed
-
•• A randomized trial that established combined nivolumab plus chemotherapy as a first line standard of care systemic therapy in advanced gastric or gastroesophageal junction cancer.
-
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4 - DOI - PubMed
-
•• A randomized trial that established combined pembrolizumab plus chemotherapy as a first line standard of care systemic therapy in advanced gastric or gastroesophageal junction cancer
-
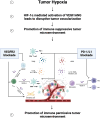
- Saeed A, Park R, Sun W.. The integration of immune checkpoint inhibitors with VEGF targeted agents in advanced gastric and gastroesophageal adenocarcinoma: a review on the rationale and results of early phase trials. J Hematol Oncol. 2021;14(1):13. doi: 10.1186/s13045-021-01034-0 - DOI - PMC - PubMed
-
•• A review of the current clinical and translational research in and rationale for combined VEGF and PD-1/L1 inhibition in gastric or gastroesophageal junction cancer.
-
- Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559 - DOI - PMC - PubMed
-
• A preclinical study showing that VEGF-A modulates T cell exhaustion, demonstrating preclinical rationale for the combination of VEGF and PD-1 inhibition.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
