Pharmacokinetics and Safety of Levofloxacin for Treatment of Rifampicin-Resistant Tuberculosis During Pregnancy and the Postpartum Period: Results from IMPAACT P1026s
- PMID: 40155501
- PMCID: PMC12041113
- DOI: 10.1007/s40262-025-01498-0
Pharmacokinetics and Safety of Levofloxacin for Treatment of Rifampicin-Resistant Tuberculosis During Pregnancy and the Postpartum Period: Results from IMPAACT P1026s
Abstract
Background and objective: Treatment of rifampicin-resistant tuberculosis (RR-TB) often includes fluoroquinolones, but data on long-term exposure during and after pregnancy are limited. We examined the pharmacokinetics and safety of levofloxacin in an observational cohort of pregnant and postpartum women receiving treatment for RR-TB.
Methods: Participants were enrolled in their second or third trimester and underwent intensive pharmacokinetic sampling to quantify levofloxacin plasma concentrations at 20-26 weeks' and 30-38 weeks' gestation and at 2-8 weeks postpartum. The levofloxacin plasma concentration target was 7 µg/mL. Pharmacokinetic parameters over 12 and 24 h were described using non-compartmental analysis and within-participant comparison during pregnancy versus postpartum. Adverse events were extracted from medical records. Infants were enrolled in utero and followed on study for 4-6 months after birth.
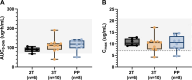
Results: A total of 11 pregnant women, with a median age of 31 years, received RR-TB treatment including levofloxacin; 6 (55%) were living with HIV. In the second trimester, third trimester, and postpartum, median maximum plasma drug concentration values were 10.3, 10.6, and 10.6 µg/mL, and area under the concentration time curve over 12 h (AUC0-12) were 69.0, 77.6, and 80.2 µg·h/mL, respectively. Compared with postpartum, median AUCs were lower and clearance was higher in the second but not the third trimester. Eight (72%) women and seven (64%) infants experienced severe or life-threatening adverse events or outcomes that were unlikely to be related to levofloxacin.
Conclusions: Levofloxacin AUC0-12 was lower in the second trimester than the third trimester of pregnancy and the postpartum period, but exposures overall were within target ranges. Further research is warranted to explore the clinical significance of these findings.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Stellenbosch University. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health, under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The University of Cape Town Clinical PK Laboratory has been granted funding as an International Pharmacology Specialty Laboratory by the IMPAACT network. Conflicts of Interest: The authors have no conflicts of interest to declare. Ethics Approval: Ethics review board approvals for the IMPAACT P1026s study were received from the Stellenbosch University Health Research Ethics Committee (N13/02/025_DTTC; 24 November 2016) and the University of the Witwatersrand Human Research Ethics Committee: Medical (160913; 24 March 2017). This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Consent to Participate: All pregnant participants provided written informed consent for themselves and their infants to take part in this study. Consent for Publication: Not applicable (data are presented in aggregate and no personally identifiable information has been included in this manuscript). Author’s Contributions: AS, BMB, MM, and DES contributed to the conceptualisation of the study and study design. RB, KG and NC contributed to the study implementation and oversight. PDK, LW, JSN, and LF contributed to the study implementation, data collection, and sample processing at the highest enrolling study sites. JH and ACE contributed to the literature search for the manuscript. KK contributed to the data extraction. JH, MP, KMB, DES, and MvS contributed to the data analysis. JH, ACE, ACH, and ED contributed to the writing of the manuscript. All authors contributed to reviewing the manuscript, and all authors have read and approved the final version. Data Availability: The data that support the findings of this study cannot be made publicly available because of the ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon reasonable request to the IMPAACT Statistical and Data Management Center’s data access committee (email: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.
Figures

References
-
- WHO. World Health Organization. Global tuberculosis report 2023. Geneva, Switzerland: WHO. 2023. https://www.who.int/teams/global-tuberculosis-programme/data, accessed 27 Nov 2023; 2023.
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. Geneva, Switzerland: WHO. 2022. https://www.who.int/publications/i/item/9789240063129 (Accessed on 22 Jul 2023); 2022.
-
- World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB), August 2018. Licence: CC BY-NC-SA 3.0 IGO. Geneva, Switzerland: WHO. 2018. https://www.who.int/publications/i/item/WHO-CDS-TB-2018.18 (Accessed 04 Oct 2023); 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

