The homozygous LRRK2.p.N1437D point mutation mouse is a novel model of parkinsonism
- PMID: 40155632
- PMCID: PMC11953335
- DOI: 10.1038/s41531-025-00905-4
The homozygous LRRK2.p.N1437D point mutation mouse is a novel model of parkinsonism
Abstract
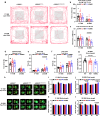
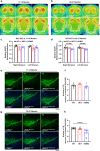
The leucine-rich repeat kinase 2 (LRRK2) gene is one of the most common genetic causes of autosomal dominant Parkinson's disease (PD) and a common genetic risk factor for sporadic PD. However, aged mice with common LRRK2 point mutations fail to exhibit age-related PD-associated behavioral and pathological impairments. We generated a novel mouse model harboring the LRRK2.p.N1437D point mutation (c.4309 A > G; NM_98578). Here, the homozygous N1437D mutation, but not the heterozygous mutation, led to an increase in the autophosphorylation, substrate phosphorylation, and GTP-binding capacity of LRRK2. Heterozygous N1437D mice also showed unaffected behavior and pathology while the homozygous mice exhibited PD-associated behavioral change at 25-26 months, dopamine system damage, lipofuscin accumulation, and lipid peroxidation in substantia nigra dopaminergic neurons at 26-27 months. The new N1437D point mutation mouse does not require LRRK2 overexpression and may better mimic the pathological characteristics of LRRK2 mutation in the ROC-COR region.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests
Figures




References
-
- Alessi, D. R. & Sammler, E. LRRK2 kinase in Parkinson’s disease. Science360, 36–37 (2018). - PubMed
Grants and funding
- 82271511/National Natural Science Foundation of China (National Science Foundation of China)
- 82201406/National Natural Science Foundation of China (National Science Foundation of China)
- 82371261/National Natural Science Foundation of China (National Science Foundation of China)
- 92249302/National Natural Science Foundation of China (National Science Foundation of China)
- 82171421/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

