Cardioprotective role of SIRT1 activation on mitochondrial function in insulin-resistant H9c2 cells
- PMID: 40155821
- PMCID: PMC11954332
- DOI: 10.1186/s12872-024-04397-7
Cardioprotective role of SIRT1 activation on mitochondrial function in insulin-resistant H9c2 cells
Abstract
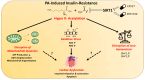
Background: Insulin-resistance in cardiomyocytes is often associated with metabolic disorders like obesity, and type2 diabetes. Studies demonstrated that sirtuin1 (SIRT1) plays a protective role in cells resistant to insulin by enhancing insulin sensitivity and improving glucose metabolism. Based on these protective functions observed in SIRT1, this study aims to investigate the roles of SIRT1 in palmitate (PA)-induced insulin-resistant H9C2 cells.
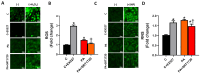
Methods: Insulin-resistance was induced in H9c2 cells via incubation with palmitic acid (50µM;24 h). Control and Insulin-resistant cells were incubated with SIRT1 inhibitor (EX527;10µM) and SIRT1 activator (SRT1720;2µM) for 24 h, respectively. Mitochondrial membrane potential (MMP), reactive oxygen/nitrogen species (ROS/RNS), total ATP production, intracellular free zinc and calcium levels ([Ca2+]i and [Zn2+]i) were monitored with fluorescence techniques. Protein levels were determined by using western-blot analysis.
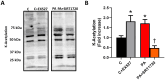
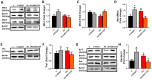
Results: K-acetylation level was increased in PA-induced Insulin-resistant cells and SIRT1 inhibited control cells. ROS/RNS production, [Ca2+]i, and [Zn2+]i levels were elevated, MMP was depolarized and ATP production was decreased in PA and EX527 treated cells compared to control cells. Mfn1 and Fis1 levels were remained unchanged, however Mfn2 protein level was elevated in cells treated with PA and SIRT1 inhibitor. Nevertheless, anti- and pro-apoptotic protein level was reduced and augmented respectively in insulin-resistant and SIRT1 inhibited cells. Activation of SIRT1 in PA-treated cells restored mitochondrial function and intracellular ionic homeostasis, reduced K-acetylation, and mitigated apoptosis.
Conclusion: Therefore, it can be proposed that the activation of SIRT1, acting as a novel regulator, may offer direct cardioprotection by restoring mitochondrial function in the insulin-resistant heart.
Keywords: Calcium; Cardiomyocytes; Insulin-resistant; Mitochondria; SIRT1; Zinc.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
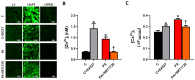

References
-
- Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–148. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

